GNE-317 Reverses MSN-Mediated Proneural-to-Mesenchymal Transition and Suppresses Chemoradiotherapy Resistance in Glioblastoma via PI3K/mTOR
- PMID: 39921260
- PMCID: PMC11948001
- DOI: 10.1002/advs.202412517
GNE-317 Reverses MSN-Mediated Proneural-to-Mesenchymal Transition and Suppresses Chemoradiotherapy Resistance in Glioblastoma via PI3K/mTOR
Abstract
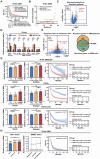
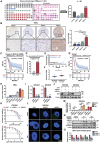
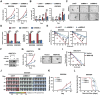
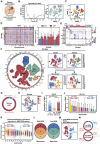
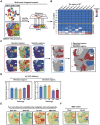
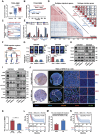
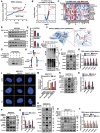
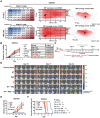
Glioblastoma (GBM) resistance to chemoradiotherapy is a major factor contributing to poor treatment outcomes. This resistance markedly affects the effectiveness of surgery combined with chemoradiotherapy and leads to post-surgical tumor recurrence. Therefore, exploring the mechanisms underlying chemoradiotherapy resistance in GBM is crucial for understanding its progression and improving therapeutic options. This study found that moesin (MSN) acts as a key promotor of chemoradiotherapy resistance in glioma stem cells (GSCs), enhancing their proliferation and stemness maintenance. Mechanistically, MSN activates the downstream PI3K/mTOR signaling pathway, driving the proneural-to-mesenchymal transition (PMT) in GSCs. This process enhances the repair of DNA damage caused by radiotherapy (RT) and temozolomide (TMZ), thereby increasing the resistance of GSCs to chemoradiotherapy. Additionally, GNE-317, a small molecule drug capable of crossing the blood-brain barrier, specifically inhibits MSN and suppresses the activation of downstream PI3K/mTOR signaling. Importantly, the combination of GNE-317 with RT and TMZ exhibits a strong synergistic effect both in vivo and in vitro, achieving better efficacy compared to the traditional combination of RT and TMZ. This study not only advances understanding of the mechanisms underlying chemoradiotherapy resistance in GBM but also provides a promising new approach for enhancing treatment outcomes.
Keywords: GNE‐317; MSN; PI3K/mTOR; chemoradiotherapy resistance; glioblastoma; proneural‐to‐mesenchymal transition.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Alexander B. M., Cloughesy T. F., Glioblastoma A., J. Clin. Oncol. 2017, 35, 2402. - PubMed
-
- Stupp R., Hegi M. E., Mason W. P., van den Bent M. J., Taphoorn M. J. B., Janzer R. C., Ludwin S. K., Allgeier A., Fisher B., Belanger K., Hau P., Brandes A. A., Gijtenbeek J., Marosi C., Vecht C. J., Mokhtari K., Wesseling P., Villa S., Eisenhauer E., Gorlia T., Weller M., Lacombe D., Cairncross J. G., Mirimanoff R.‐O., Lancet Oncol. 2009, 10, 459. - PubMed
-
- Wen P. Y., Kesari S., N. Engl. J. Med. 2008, 359, 492. - PubMed
MeSH terms
Substances
Grants and funding
- 82073276/National Natural Science Foundation of China
- 82273100/National Natural Science Foundation of China
- 82302999/National Natural Science Foundation of China
- 2022KJ229/Tianjin Education Commission scientific research plan general project
- 20YFZCSY00070/Tianjin Municipal Transportation Commission Science and Technology Development Plan Project
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
