Inhibition of Tacrolimus Metabolism by Cannabidiol and Its Metabolites In Vitro
- PMID: 39921332
- PMCID: PMC11806196
- DOI: 10.1111/cts.70152
Inhibition of Tacrolimus Metabolism by Cannabidiol and Its Metabolites In Vitro
Abstract
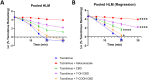
Drug interactions are major causes of interindividual variability in tacrolimus exposure and effect. Tacrolimus, a widely used drug in transplant patients, is metabolized by CYP3A4 and CYP3A5. Cannabidiol (CBD) use after transplant is common. Clinical cases suggest CBD may alter tacrolimus exposure, but the mechanism of this interaction is unknown. We hypothesize that cannabidiol will inhibit tacrolimus metabolism in vitro mainly through CYP3A5 inhibition. In pooled human liver microsomes (HLMs) and recombinant (r) CYP3A4 and CYP3A5 enzymes, tacrolimus (1 μM) metabolism was determined using substrate depletion method in the absence (control) and the presence of 10 μM CBD, 7-hydroxyCBD, and 7-carboxyCBD. Ketoconazole (1 μM) served as a positive control for the inhibition of CYP3A. Linear regression analyses were performed to obtain kinetic parameters of the depletion. Tacrolimus depletion half-life was 2.54, 0.922, and 0.351 min with pooled HLMs, rCYP3A4, and rCYP3A5, respectively. In pooled HLMs, CBD and 7-hydroxyCBD increased tacrolimus half-life by 0.8- and 2.3-fold (both p < 0.0001), respectively. In rCYP3A4, CBD, 7-hydroxyCBD, and ketoconazole prolonged tacrolimus half-life by 5.8-, 14-, and 7.7-fold, respectively. In rCYP3A5, CBD, 7-hydroxyCBD, and ketoconazole prolonged half-life by 29.3-, 19.7-, and 0.1-fold, respectively. In all experiments, 7-carboxyCBD had minimal effect on tacrolimus depletion. CBD and 7-hydroxyCBD inhibited tacrolimus metabolism in vitro. CBD showed stronger inhibition in rCYP3A5 than rCYP3A4. The demonstrated CYP3A5 selectivity of cannabidiol may contribute to the in vitro identification of CYP3A5 substrates in new drug development. Our results support the potential of a clinical drug-drug interaction between CBD and tacrolimus.
Keywords: CYP; cannabinoid; drug–drug interactions; pharmacokinetics.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- OPTN/SRTR 2022 Annual Data Report , “Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR),” accessed March 1, 2024, http://srtr.transplant.hrsa.gov/annual_reports/Default.aspx.
-
- Kelly D. A., Bucuvalas J. C., Alonso E. M., et al., “Long‐Term Medical Management of the Pediatric Patient After Liver Transplantation: 2013 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation,” Liver Transplantation 19, no. 8 (2013): 798–825, 10.1002/lt.23697. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

