NEAT1-mediated regulation of proteostasis and mRNA localization impacts autophagy dysregulation in Rett syndrome
- PMID: 39921568
- PMCID: PMC11806351
- DOI: 10.1093/nar/gkaf074
NEAT1-mediated regulation of proteostasis and mRNA localization impacts autophagy dysregulation in Rett syndrome
Abstract
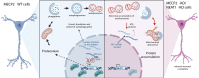
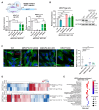
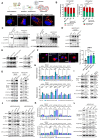
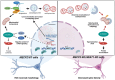
Rett syndrome (RTT) is a severe neurodevelopmental disorder primarily caused by loss-of-function mutations in the MECP2 gene, resulting in diverse cellular dysfunctions. Here, we investigated the role of the long noncoding RNA (lncRNA) NEAT1 in the context of MeCP2 deficiency using human neural cells and RTT patient samples. Through single-cell RNA sequencing and molecular analyses, we found that NEAT1 is markedly downregulated in MECP2 knockout (KO) cells at various stages of neural differentiation. NEAT1 downregulation correlated with aberrant activation of the mTOR pathway, abnormal protein metabolism, and dysregulated autophagy, contributing to the accumulation of protein aggregates and impaired mitochondrial function. Reactivation of NEAT1 in MECP2-KO cells rescued these phenotypes, indicating its critical role downstream of MECP2. Furthermore, direct RNA-RNA interaction was revealed as the key process for NEAT1 influence on autophagy genes, leading to altered subcellular localization of specific autophagy-related messenger RNAs and impaired biogenesis of autophagic complexes. Importantly, NEAT1 restoration rescued the morphological defects observed in MECP2-KO neurons, highlighting its crucial role in neuronal maturation. Overall, our findings elucidate lncRNA NEAT1 as a key mediator of MeCP2 function, regulating essential pathways involved in protein metabolism, autophagy, and neuronal morphology.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

