In Vitro Discovery of a Therapeutic Lead for HFMD From a Library Screen of Rocaglates/Aglains
- PMID: 39921602
- PMCID: PMC11806654
- DOI: 10.1002/jmv.70228
In Vitro Discovery of a Therapeutic Lead for HFMD From a Library Screen of Rocaglates/Aglains
Abstract
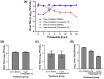
The lack of effective antiviral treatments for enteroviruses, including human enterovirus A71 (EV-A71), have resulted in an immense global healthcare burden associated with hand-foot-and-mouth disease (HFMD). Rocaglates and aglains belong to a family of compounds produced by Aglaia genus plants. Since the initial discovery of rocaglates in 1982, various rocaglates and aglains have been synthesized and extensively studied mainly as anticancer agents. Here, we report the discovery of a novel aglain derivative as a potential EV-A71 inhibitor. From an immunofluorescence-based phenotypic screen of a library of 296 rocaglate and aglain derivatives, we identified a lead aglain which effectively suppressed EV-A71 replication by 2.3 log fold at a non-cytotoxic concentration, with a host cell CC50 of 21.78 µM, an EV-A71 infection EC50 of 3.57 µM, and a selectivity index of 6.1. Further validation revealed inhibition of EV-A71 across multiple human cell types and a pan-enterovirus inhibitory spectrum against other enteroviruses. Subsequent mechanistic investigation revealed interference with EV-A71 intracellular post-entry events including viral RNA transcription and translation. Findings from this study have established a strong foundation for development of aglain scaffolds as much needed antiviral agents for HFMD, paving the way for future medicinal chemistry optimization and in vivo studies.
Keywords: aglain; antiviral agents; enterovirus; hand, foot, and mouth disease; natural products; pentafluorophenyl.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare the following competing financial interest(s): J.J. H.C., A.O., W.W., L.E.B., and J.A.P., Jr. are named as inventors on a U.S. provisional patent application pertaining to the findings reported here.
Figures





References
MeSH terms
Substances
Grants and funding
- R35 GM118173/GM/NIGMS NIH HHS/United States
- S10 OD028585/OD/NIH HHS/United States
- U01 TR002625/TR/NCATS NIH HHS/United States
- This work was supported by Singapore National Research Foundation Competitive Research Programme (NRF-CRP21-2018-0004) (SA & JJHC), Singapore Ministry of Education (MOE) Academic Research Fund (AcRF) Tier 2 (MOE-T2EP30221-0005) (JJHC), and NIH grants R35GM118173 and U01TR002625 (JAP, Jr.). We thank the National Science Foundation for support of NMR (CHE-0619339) and MS (CHE-0443618) facilities at BU, and the NIH (S10OD028585) for support of the single-crystal XRD system.
LinkOut - more resources
Full Text Sources

