Perivascular glial reactivity is a feature of phosphorylated tau lesions in chronic traumatic encephalopathy
- PMID: 39921702
- PMCID: PMC11807024
- DOI: 10.1007/s00401-025-02854-x
Perivascular glial reactivity is a feature of phosphorylated tau lesions in chronic traumatic encephalopathy
Abstract
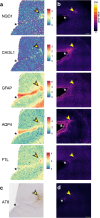
Chronic traumatic encephalopathy (CTE), a neurodegenerative disease associated with repetitive head injuries, is characterised by perivascular hyperphosphorylated tau (p-tau) accumulations within the depths of cortical sulci. Although the majority of CTE literature focuses on p-tau pathology, other pathological features such as glial reactivity, vascular damage, and axonal damage are relatively unexplored. In this study, we aimed to characterise these other pathological features, specifically in CTE p-tau lesion areas, to better understand the microenvironment surrounding the lesion. We utilised multiplex immunohistochemistry to investigate the distribution of 32 different markers of cytoarchitecture and pathology that are relevant to both traumatic brain injury and neurodegeneration. We qualitatively assessed the multiplex images and measured the percentage area of labelling for each marker in the lesion and non-lesion areas of CTE cases. We identified perivascular glial reactivity as a prominent feature of CTE p-tau lesions, largely driven by increases in astrocyte reactivity compared to non-lesion areas. Furthermore, we identified astrocytes labelled for both NAD(P)H quinone dehydrogenase 1 (NQO1) and L-ferritin, indicating that lesion-associated glial reactivity may be a compensatory response to iron-induced oxidative stress. Our findings demonstrate that perivascular inflammation is a consistent feature of the CTE pathognomonic lesion and may contribute to the pathogenesis of brain injury-related neurodegeneration.
Keywords: Astrocyte; Brain injury; Chronic traumatic encephalopathy; Neurodegeneration; Neuroinflammation; Neuropathology; Tau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors have no competing interests to declare that are relevant to the content of this article.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

