The prognostic and predictive value of the luminal-like subtype in hormone receptor-positive breast cancer: an analysis of the DATA trial
- PMID: 39921934
- PMCID: PMC11850755
- DOI: 10.1016/j.esmoop.2025.104154
The prognostic and predictive value of the luminal-like subtype in hormone receptor-positive breast cancer: an analysis of the DATA trial
Abstract
Background: This study determines the prognostic value of the luminal-like subtype in patients with hormone receptor-positive breast cancer and explores whether the efficacy of extended anastrozole therapy differs between patients with luminal A-like versus luminal B-like tumours.
Materials and methods: The phase III DATA study (NCT00301457) examined the efficacy of 6 versus 3 years of anastrozole in postmenopausal women with early-stage hormone receptor-positive breast cancer who had received 2-3 years of tamoxifen. Patients with available formalin-fixed paraffin-embedded tissue blocks were identified and classified by immunohistochemical luminal-like subtype. Distant recurrence (DR) and breast cancer-specific mortality (BCSM) were compared by luminal-like subtype and treatment arm using competing risk methods.
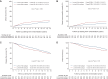
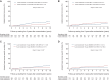
Results: This study included 788 patients: 491 had a luminal A-like tumour and 297 had a luminal B-like tumour. The median follow-up time was 13.1 years. Patients with luminal B-like tumours experienced a higher risk of DR [subdistribution hazard ratio (sHR) 1.44, 95% confidence interval (CI) 1.03-2.01, P = 0.03] and BCSM (sHR 1.68, 95% CI 1.15-2.45, P = 0.008) than patients with luminal A-like tumours. The efficacy of extended anastrozole therapy differed between patients with luminal A-like tumours (DR: sHR 0.51, 95% CI 0.30-0.88, P = 0.02; BCSM: sHR 0.39, 95% CI 0.19-0.82, P = 0.01) and patients with luminal B-like tumours (DR: sHR 2.09, 95% CI 0.96-4.53, P = 0.06; BCSM: sHR 2.36, 95% CI 0.80-7.00, P = 0.12) (P-interaction = 0.03 and P-interaction = 0.06, respectively).
Conclusion: In patients with hormone receptor-positive breast cancer, the luminal B-like subtype was associated with a significantly worse prognosis when compared with the luminal A-like subtype. Extended anastrozole therapy halved the risk of DR and BCSM in patients with luminal A-like tumours, whereas no effect was seen in patients with luminal B-like tumours.
Keywords: Ki-67 antigen; aromatase inhibitors; breast neoplasms; luminal-like subtype; prognosis.
Copyright © 2025 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Bardou V.J., Arpino G., Elledge R.M., Osborne C.K., Clark G.M. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21(10):1973–1979. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

