Molecular basis of convergent evolution of ACE2 receptor utilization among HKU5 coronaviruses
- PMID: 39922192
- PMCID: PMC12237432
- DOI: 10.1016/j.cell.2024.12.032
Molecular basis of convergent evolution of ACE2 receptor utilization among HKU5 coronaviruses
Abstract
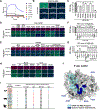
DPP4 was considered a canonical receptor for merbecoviruses until the recent discovery of African bat-borne MERS-related coronaviruses using ACE2. The extent and diversity of ACE2 utilization among merbecoviruses and their receptor species tropism remain unknown. Here, we reveal that HKU5 enters host cells utilizing Pipistrellus abramus (P.abr) and several non-bat mammalian ACE2s through a binding mode distinct from that of any other known ACE2-using coronaviruses. We defined the molecular determinants of receptor species tropism and identified a single amino acid mutation enabling HKU5 to utilize human ACE2, providing proof of principle for machine-learning-assisted outbreak preparedness. We show that MERS-CoV and HKU5 have markedly distinct antigenicity and identified several HKU5 inhibitors, including two clinical compounds. Our findings profoundly alter our understanding of coronavirus evolution, as several merbecovirus clades independently evolved ACE2 utilization, and pave the way for developing countermeasures against viruses poised for human emergence.
Keywords: HKU5; MERS-CoV; antibodies; coronaviruses; merbecoviruses; spillover; viral receptor.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.Y. has submitted a patent application to the China National Intellectual Property Administration for the utilization of propagation-competent VSV to evaluate amplification of ACE2-using merbecoviruses in bat ACE2-expressing cells.
Figures




Update of
-
Molecular basis of convergent evolution of ACE2 receptor utilization among HKU5 coronaviruses.bioRxiv [Preprint]. 2024 Aug 28:2024.08.28.608351. doi: 10.1101/2024.08.28.608351. bioRxiv. 2024. Update in: Cell. 2025 Mar 20;188(6):1711-1728.e21. doi: 10.1016/j.cell.2024.12.032. PMID: 39253417 Free PMC article. Updated. Preprint.
References
-
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA, et al. (2003). Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1967–1976. - PubMed
-
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W, et al. (2003). A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1953–1966. - PubMed
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, and Fouchier RA (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367, 1814–1820. - PubMed
-
- Mok CKP, Zhu A, Zhao J, Lau EHY, Wang J, Chen Z, Zhuang Z, Wang Y, Alshukairi AN, Baharoon SA, et al. (2020). T-cell responses to MERS coronavirus infection in people with occupational exposure to dromedary camels in Nigeria: an observational cohort study. Lancet Infect. Dis. 10.1016/S1473-3099(20)30599-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

