Temporal trends in guideline-recommended medical therapy after an acute heart failure decompensation event: an observational analysis from Generator Heart Failure DataMart
- PMID: 39922597
- PMCID: PMC11808906
- DOI: 10.1136/bmjopen-2024-088998
Temporal trends in guideline-recommended medical therapy after an acute heart failure decompensation event: an observational analysis from Generator Heart Failure DataMart
Abstract
Objectives: To evaluate the trend of prescription of the four foundational therapies, and their impact on 30-day urgent re-admissions and all-cause death in patients with heart failure and reduced ejection fraction (HFrEF) following an acute decompensation event.
Design: Retrospective.
Setting: One tertiary referral centre.
Participants: 999 consecutively patients admitted with a primary diagnosis of HFrEF between January 2020 and June 2023 were identified through a validated, high-performance technology infrastructure based on artificial intelligence. The entire cohort was divided into three time periods based on two time points: September 2021 (ie, the release of the latest European guidelines) and January 2022 (ie, reimbursement for sodium-glucose cotransporter 2 (SGLT2) inhibitors).
Primary and secondary outcome measures: Trends and predictors of the prescription of each of the four foundational therapies and of the composite of all-cause death and rehospitalisation for urgent causes at 30 days.
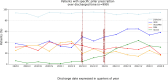
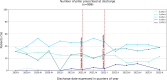
Results: Among the 999 included patients, β-blockers were prescribed in 93% of patients, ACE inhibitor (ACEi)/angiotensin receptor blocker (ARB)/angiotensin-neprilysin receptor inhibitor (ARNi) in 73%, mineralocorticoid receptor antagonist in 30% and SGLT2 inhibitors in 18%. Over time, an increase in the prescription rate occurred only for SGLT2 inhibitors (3% vs 10% vs 32%, p<0.001), whereas the rate of the composite of all-cause death and rehospitalisation for urgent causes at 30 days remained stable (9.9% vs 10.3% vs 8.4%; p=ns). In multivariate analysis, the use of ACEi/ARB/ARNi was associated with a lower risk of 30-day all-cause death and urgent rehospitalisation (adjusted OR 0.38; 95% CI 0.24 to 0.59; p<0.01). Conversely, the prescription of furosemide at discharge (adjusted OR 2.25; 95% CI 95% 1.29 to 3.94; p<0.01) and a previous genitourinary infection (adjusted OR 4.02; 95% CI 1.67 to 9.68; p<0.01) were associated with higher risk of 30-day all-cause death and urgent rehospitalisation.
Conclusions: In our study, early adoption of guideline-recommended medical therapy is still limited, with a significant rise in SGLT2i prescriptions after January 2022 and a lower risk of the composite of all-cause death and urgent readmissions at 30 days restricted to the use of ACEi/ARB/ARNi.
Keywords: CLINICAL PHARMACOLOGY; Heart failure; Hospitalization.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: GSavarese reports grants and personal fees from CSL Vifor, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Cytokinetics, Pharmacosmos, Medtronic, Bayer and personal fees from Roche, Abbott, Edwards Lifesciences, TEVA, INTAS, Menarini, Hikma and grants from Boston Scientific, Merck, all outside the submitted work. The remaining authors have no conflicts of interest to declare for the present work.
Figures


References
-
- Laborante R, Savarese G, Patti G, et al. Safety and efficacy of early initiation of sodium-glucose cotransporter-2 inhibitors after an acute coronary syndrome event: a meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2024;10:646–8. doi: 10.1093/ehjcvp/pvae047. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
