Genomics of pediatric cardiomyopathy
- PMID: 39922924
- PMCID: PMC12106076
- DOI: 10.1038/s41390-025-03819-2
Genomics of pediatric cardiomyopathy
Abstract
Cardiomyopathy in children is a leading cause of heart failure and cardiac transplantation. Disease-associated genetic variants play a significant role in the development of the different subtypes of disease. Genetic testing is increasingly being recognized as the standard of care for diagnosing this heterogeneous group of disorders, guiding management, providing prognostic information, and facilitating family-based risk stratification. The increase in clinical and research genetic testing within the field has led to new insights into this group of disorders. Mutations in genes encoding sarcomere, cytoskeletal, Z-disk, and sarcolemma proteins appear to play a major role in causing the overlapping clinical phenotypes called cardioskeletal myopathies through "final common pathway" links. For myocarditis, the high frequency of infectious exposures and wide spectrum of presentation suggest that genetic factors mediate the development and course of the disease, including genetic risk alleles, an association with cardiomyopathy, and undiagnosed arrhythmogenic cardiomyopathy. Finally, while we have made strides in elucidating the genetic architecture of pediatric cardiomyopathy, understanding the clinical implications of variants of uncertain significance remains a major issue. The need for continued genetic innovation in this field remains great, particularly as a basis to drive forward targeted precision medicine and gene therapy efforts. IMPACT: Cardiomyopathy and skeletal myopathy can occur in the same patient secondary to gene mutations that encode for sarcomeric or cytoskeletal proteins, which are expressed in both muscle groups, highlighting that there are common final pathways of disease. The heterogeneous presentation of myocarditis is likely secondary to a complex interaction of multiple environmental and genetic factors, suggesting a utility to genetic testing in pediatric patients with myocarditis, particularly those in higher risk groups. Given the high prevalence of variants of uncertain significance in genetic testing, better bioinformatic tools and pipelines are needed to resolve their clinical meaning.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.L. reports being the chair of the Children’s Cardiomyopathy Foundation medical advisory board and their Chief Medical Officer. S.L. is also on the medical advisory board of Secretome Therapeutics, was also a Bayer consultant, and a member of the Roche Data Safety Monitoring Board. S.L. has also served in the following editorial roles: American College of Cardiology (Editor), Elsevier (Editor), Biomed Central (Editor), American Heart Association (Scientific Statement Chair).
Figures
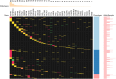
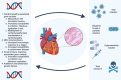
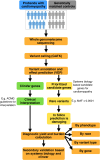
References
-
- Hershberger, R. E. et al. Genetic Evaluation of Cardiomyopathy: A Clinical Practice Resource of the American College of Medical Genetics and Genomics (Acmg). Genet. Med.20, 899–909 (2018). - PubMed
-
- Towbin, J. A. et al. Incidence, Causes, and Outcomes of Dilated Cardiomyopathy in Children. JAMA296, 1867–1876 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
- U01 HD052104/HD/NICHD NIH HHS/United States
- R01 HL087000/HL/NHLBI NIH HHS/United States
- F31 HL094100/HL/NHLBI NIH HHS/United States
- R01 CA127642/CA/NCI NIH HHS/United States
- R01 HL095127/HL/NHLBI NIH HHS/United States
- R01 HL078522/HL/NHLBI NIH HHS/United States
- K30 HL004537/HL/NHLBI NIH HHS/United States
- T32 HL007188/HL/NHLBI NIH HHS/United States
- R01 HL053392/HL/NHLBI NIH HHS/United States
- R01 HL072705/HL/NHLBI NIH HHS/United States
- U01 HD052102/HD/NICHD NIH HHS/United States
- R13 HL087708/HL/NHLBI NIH HHS/United States
- U01 AI050274/AI/NIAID NIH HHS/United States
- P01 CA068484/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

