Early COVID-19 and protection from Omicron in a highly vaccinated population in Ontario, Canada: a matched prospective cohort study
- PMID: 39923009
- PMCID: PMC11806543
- DOI: 10.1186/s12879-024-10331-1
Early COVID-19 and protection from Omicron in a highly vaccinated population in Ontario, Canada: a matched prospective cohort study
Abstract
Objectives: Predictions regarding the on-going burden of SARS-CoV-2, and vaccine recommendations, require an understanding of infection-associated immune protection. We assessed whether early COVID-19 provided protection against Omicron infection.
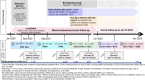
Methods: We enrolled a cohort of adults in Ontario, Canada, with COVID-19 prior to October 2020 (early infection, EI), and a matched cohort with COVID-19 testing and a negative PCR (non-EI). Participants completed baseline surveys then surveys every two weeks until January 2023. Multivariable Cox regression was used to assess factors associated with COVID-19 infection during the first 14 months of Omicron.
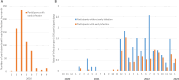
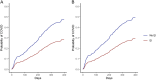
Results: Overall, 624 EI (70%) and 175 (77%) non-EI participants met criteria for analysis; 590 (95%) EI and 164 (94%) non-EI had received at least 2 COVID-19 vaccine doses prior to Omicron. Of 624 EI, 175 (28%) had one SARS-CoV-2 re-infection and 8 (1.3%) had two, compared to 84 (48%) non-EI participants with one, 5 (2.9%) with two and 1 (0.6%) with 3 infections (P < 0.0001). In multivariable analysis of risk factors for Omicron infection, the overall hazard ratio (HR, 95%CI) associated with EI was 0.56 (0.43-0.74); HRs for BA.1/2, BA.4/5 and mixed BA.5/BQ.1/XBB periods were 0.66 (0.45-0.97), 0.44 (0.28-0.68) and 0.71 (0.32-1.56). EI and BA.1/2 infection combined reduced later Omicron infection (HR 0.07 (0.03-0.21) compared to no prior infection. Older age, non-White ethnicity, no children in household, and lower neighbourhood income were associated with reduced risk of infection.
Conclusions: In our highly vaccinated population, early SARS-CoV-2 infection was associated with a 44% reduction in symptomatic COVID-19 during the first 14 months of Omicron, providing significant protection against re-infection for more than 2 years.
Keywords: COVID-19; Re-infection; SARS-CoV-2 infection; Vaccination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed consent was obtained from all study participants. The study was approved by the research ethics boards of participating hospitals (North York General #2024-0233-1025; Sunnybrook Health Sciences Centre #149–1994; Michael Garron Hospital #084-0209-Lab-01, University Health Network #14-8339-AE, The Scarborough Health Network #MED-02-011, William Osler Health System #95 − 0001, and Mount Sinai Hospital #21-0069-E). Consent for publication: Not applicable. Competing interests: MHK, CM, MM, PZ, JV, JY and JMM are employees and shareholders of Pfizer Inc. AM declares grants and personal fees from Pfizer, grants from Sanofi, personal fees from AstraZeneca, GlaxoSmithKline, Moderna and Novavax, outside the submitted work. ACG has received research funds from Providence Therapeutics Holdings, Inc., outside the submitted work. All other authors, no conflicts of interest.
Figures

References
-
- Reiner RC, Collins JK, Murray CJL. Forecasting the trajectory of the COVID-19 pandemic into 2023 under plausible variant and intervention scenarios: a global modelling study. medRxiv, 10.1101/2023030723286952. 2023. - DOI
-
- World Health Organization. Coronavirus (COVID-19) Dashboard. https://covid19.who.int/. Accessed August 6, 2023.
-
- World Health Organization. Interim statement on hybrid immunity and increasing population seroprevalence rates. June 1. 2022. https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-imm....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

