Congestion Enriches Intra-hepatic Macrophages Through Reverse Zonation of CXCL9 in Liver Sinusoidal Endothelial Cells
- PMID: 39923846
- PMCID: PMC12198043
- DOI: 10.1016/j.jcmgh.2025.101475
Congestion Enriches Intra-hepatic Macrophages Through Reverse Zonation of CXCL9 in Liver Sinusoidal Endothelial Cells
Abstract
Background & aims: Congestion alters the microenvironment of the liver sinusoid along the portal-central axis. We studied spatial changes in immune cells in the sinusoid that contribute to congestive fibrosis and portal hypertension (PHTN).
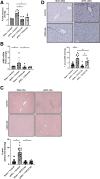
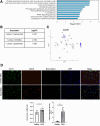
Methods: To visualize the distribution of immune cells in congestive hepatopathy (CH), we performed imaging mass cytometry (IMC) on liver tissue from patients with CH, Fontan-associated liver disease (FALD), and controls. We performed partial ligation of the inferior vena cava (pIVCL) to simulate CH in mice and isolated primary liver cells for single-cell RNA-sequencing (scRNA-seq) to study zonation of liver sinusoidal endothelial cells (LSECs). After pIVCL, mice were treated with intraperitoneal injections of AMG487, an inhibitor of the CXCL9 receptor, or a neutralizing antibody to CXCL9.
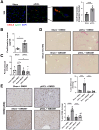
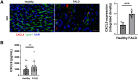
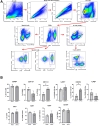
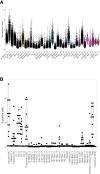
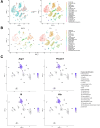
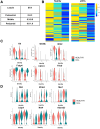
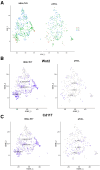
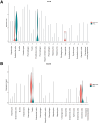
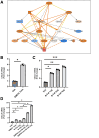
Results: Intra-hepatic macrophages are enriched in CH and FALD. Given the role of CXCL9 in macrophage patterning in the liver, we performed RNA in situ hybridization (RNAish) in CH and determined that CXCL9 was highly expressed in LSECs in FALD, suggesting that LSECs recruit macrophages in CH. After pIVCL, treatment with AMG487 or an antibody to CXCL9 attenuated portal pressures, fibrosis, and intra-hepatic macrophages. To study changes in LSECs that promote macrophage chemotaxis, we performed scRNA-seq after pIVCL and sham procedures. Analysis revealed 3 LSEC subpopulations according to sinusoidal location. RNAish identified peri-central LSECs as the predominant source of CXCL9 in FALD. In vitro analyses revealed that β-catenin and hypoxia inducible factor-1 α regulate CXCL9 transcription in peri-central LSECs.
Conclusions: CXCL9 derived from peri-central LSECs enriches intra-hepatic macrophages in CH and FALD, contributing to congestive fibrosis and PHTN. Strategies to target LSEC-derived CXCL9 may prevent the progression of CH and FALD.
Keywords: Fibrosis; Fontan-associated Liver Disease; Hypoxia; Imaging Mass Cytometry; Portal Hypertension.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Figures







References
-
- Ramachandran P., Matchett K.P., Dobie R., et al. Single-cell technologies in hepatology: new insights into liver biology and disease pathogenesis. Nat Rev Gastroenterol Hepatol. 2020;17:457–472. - PubMed
-
- Wanless I.R., Wong F., Blendis L.M., et al. Hepatic and portal vein thrombosis in cirrhosis: possible role in development of parenchymal extinction and portal hypertension. Hepatology. 1995;21:1238–1247. - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK059615/DK/NIDDK NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- K08 DK127064/DK/NIDDK NIH HHS/United States
- KL2 TR001879/TR/NCATS NIH HHS/United States
- S10 OD023465/OD/NIH HHS/United States
- T32 DK124190/DK/NIDDK NIH HHS/United States
- P30 DK084567/DK/NIDDK NIH HHS/United States
- R01 AA021171/AA/NIAAA NIH HHS/United States
- KL2 TR002379/TR/NCATS NIH HHS/United States
- R37 AA021171/AA/NIAAA NIH HHS/United States
- R56 DK059615/DK/NIDDK NIH HHS/United States
- P30 CA016520/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

