Relevance of Recent Thymic Emigrants Following Allogeneic Hematopoietic Cell Transplantation for Pediatric Patients with Inborn Errors of Immunity
- PMID: 39923938
- PMCID: PMC11957927
- DOI: 10.1016/j.jtct.2025.02.003
Relevance of Recent Thymic Emigrants Following Allogeneic Hematopoietic Cell Transplantation for Pediatric Patients with Inborn Errors of Immunity
Abstract
Background: Allogeneic hematopoietic cell transplantation (HCT) can be curative for many inborn errors of immunity (IEI). Timely neothymopoiesis is paramount to favorable clinical outcomes after HCT. Neothymopoiesis can be quantified by flow cytometric measurement of circulating recent thymic emigrants (RTE; CD31+CD4+CD45RA+ T cells).
Objectives: We hypothesized that decreased RTE would be associated with baseline HCT characteristics of older age at time of HCT and exposure to greater HCT conditioning intensity, as well as with HCT outcomes including mixed (<95%) lymphoid donor chimerism and presence of acute graft-versus-host disease (GvHD).
Study design: In this retrospective analysis two cohorts of pediatric IEI HCT recipients were identified at two centers that collected RTE data following allogeneic HCT. For both cohorts, patient and HCT information was recorded including but not limited to patient age, lymphoid donor chimerism, and occurrence of acute GvHD. Mixed effects models were fitted for the repeated measures of RTE with these covariates and time.
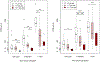
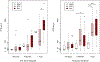
Results: Between 2012 and 2021, a total of 162 pediatric IEI HCT recipients transplanted across both cohorts were eligible for inclusion. Cohort A (n = 34) included 23 males (68%). Median age at HCT was 2.2 years (interquartile range (IQR) 0.8 to 10.8). Eight (23.5%) underwent reduced intensity (RIC), 23 (67.7%) reduced toxicity myeloablative (RTC), and 3 (8.8%) myeloablative (MAC) conditioning. All received alemtuzumab serotherapy. Cohort B (n = 128) included 87 males (68%). Median age at HCT was 1.4 years (IQR 0.7 to 5.3). Seventy-six (59.4%) underwent RIC, 38 (29.7%) RTC, and 14 (10.9%) MAC. RIC and RTC patients received alemtuzumab serotherapy, MAC antithymocyte globulin. In the linear mixed effect model for RTE at 1 year after HCT for Cohort A, significant negative associations included increasing age (P < .0001) and RTC compared to RIC (P < .01). In the linear mixed effects model for RTE at 1 year after HCT for Cohort B, significant negative associations included increasing age (P < .0001), grade 2 to 4 acute GvHD (compared to grade 0 to 1; P < .01), MAC compared to RIC (P < .0001), MAC compared to RTC (P < .01), and RTC compared to RIC (P = .03).
Conclusions: Serial measurement of RTE is a useful assessment of thymic function after HCT. In pediatric patients with IEI, older age at transplantation, greater intensity of conditioning, and occurrence of grade 2 to 4 acute GvHD were strongly associated with slower thymic-derived immune reconstitution. Mixed lymphoid donor chimerism was not associated with RTE in the linear mixed effects model. In addition to augmenting current anticipatory guidance on HCT outcomes, these findings may guide personalization of regimens to optimize clinical outcomes in IEI HCT.
Keywords: Immune reconstitution; Neothymopoiesis; Recent thymic emigrants; Reduced intensity conditioning.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
CLE has performed advisory board or consulting work for Chiesi, Ensoma Inc., Maro Bio, and Elixirgen Therapeutics.
RSA has received an investigator-initiated grant from Amgen and served as a consultant/ advisory board member for Amgen and Sobi.
Figures

References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

