Src inhibition potentiates MCL-1 antagonist activity in acute myeloid leukemia
- PMID: 39924517
- PMCID: PMC11808118
- DOI: 10.1038/s41392-025-02125-x
Src inhibition potentiates MCL-1 antagonist activity in acute myeloid leukemia
Abstract
The importance of MCL-1 in leukemogenesis has prompted development of MCL-1 antagonists e.g., S63845, MIK665. However, their effectiveness in acute myeloid leukemia (AML) is limited by compensatory MCL-1 accumulation via the ubiquitin proteasome system. Here, we investigated mechanisms by which kinase inhibitors with Src inhibitory activity e.g., bosutinib (SKI-606) might circumvent this phenomenon. MCL-1 antagonist/SKI-606 co-administration synergistically induced apoptosis in diverse AML cell lines. Consistently, Src or MCL-1 knockdown with shRNA markedly sensitized cells to MCL-1 inhibitors or SKI-606 respectively, while ectopic MCL-1 expression significantly diminished apoptosis. Mechanistically, MCL-1 antagonist exposure induced MCL-1 up-regulation, an event blocked by Src inhibitors or Src shRNA knock-down. MCL-1 down-regulation was associated with diminished transcription and increased K48-linked degradative ubiquitination. Enhanced cell death depended functionally upon down-regulation of phosphorylated STAT3 (Tyr705/Ser727) and cytoprotective downstream targets c-Myc and BCL-xL, as well as BAX/BAK activation, and NOXA induction. Importantly, the Src/MCL-1 inhibitor regimen robustly killed primary AML cells, including primitive progenitors, but spared normal hematopoietic CD34+ cells and human cardiomyocytes. Notably, the regimen significantly improved survival in an MV4-11 cell xenograft model, while reducing tumor burden in two patient-derived xenograft (PDX) AML models and increased survival in a third. These findings argue that Src inhibitors such as SKI-606 potentiate MCL-1 antagonist anti-leukemic activity in vitro and in vivo by blocking MCL-1 antagonist-mediated cytoprotective MCL-1 accumulation by promoting degradative ubiquitination, disrupting STAT-3-mediated transcription, and inducing NOXA-mediated MCL-1 degradation. They also suggest that this strategy may improve MCL-1 antagonist efficacy in AML and potentially other malignancies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All animal studies were performed under protocol AM10204, approved by our local IACUC, and regulated by VCU’s Animal Care and Use Program, in accordance with AAALAC, USDA, and PHS guidelines, and all studies were obtained with written informed consent from all participating patients undergoing routine diagnostic aspirations, were conducted in accordance with recognized ethical guidelines (e.g., the Declaration of Helsinki), and were approved by the Virginia Commonwealth Institutional Review Board (#MCC-8712-3A; MCC-02447; MCC-03340).
Figures
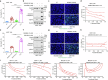




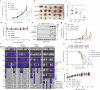

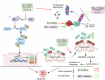
References
-
- Khwaja, A. et al. Acute myeloid leukaemia. Nat. Rev. Dis. Prim.2, 16010 (2016). - PubMed
-
- Deschler, B. & Lubbert, M. Acute myeloid leukemia: epidemiology and etiology. Cancer107, 2099–2107 (2006). - PubMed
-
- Kayser, S. & Levis, M. J. Updates on targeted therapies for acute myeloid leukaemia. Br. J. Haematol.196, 316–328 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

