AXL signaling in cancer: from molecular insights to targeted therapies
- PMID: 39924521
- PMCID: PMC11808115
- DOI: 10.1038/s41392-024-02121-7
AXL signaling in cancer: from molecular insights to targeted therapies
Abstract
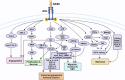
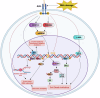
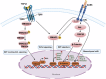
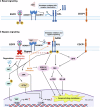
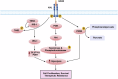
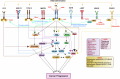
AXL, a member of the TAM receptor family, has emerged as a potential target for advanced-stage human malignancies. It is frequently overexpressed in different cancers and plays a significant role in various tumor-promoting pathways, including cancer cell proliferation, invasion, metastasis, epithelial-mesenchymal transition (EMT), angiogenesis, stemness, DNA damage response, acquired therapeutic resistance, immunosuppression, and inflammatory responses. Beyond oncology, AXL also facilitates viral infections, including SARS-CoV-2 and Zika highlighting its importance in both cancer and virology. In preclinical models, small-molecule kinase inhibitors targeting AXL have shown promising anti-tumorigenic potential. This review primarily focuses on the induction, regulation and biological functions of AXL in mediating these tumor-promoting pathways. We discuss a range of therapeutic strategies, including recently developed small-molecule tyrosine kinase inhibitors (TKIs), monoclonal antibodies, and antibody-drug conjugates (ADCs), anti-AXL-CAR, and combination therapies. These interventions are being examined in both preclinical and clinical studies, offering the potential for improved drug sensitivity and therapeutic efficacy. We further discuss the mechanisms of acquired therapeutic resistance, particularly the crosstalk between AXL and other critical receptor tyrosine kinases (RTKs) such as c-MET, EGFR, HER2/HER3, VEGFR, PDGFR, and FLT3. Finally, we highlight key research areas that require further exploration to enhance AXL-mediated therapeutic approaches for improved clinical outcomes.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Dr. Choueiri reports institutional and/or personal, paid and/or unpaid support for research, advisory boards, consultancy, and/or honoraria past 5 years, ongoing or not, from: Alkermes, Arcus Bio, AstraZeneca, Aravive, Aveo, Bayer, Bristol Myers-Squibb, Calithera, Circle Pharma, Deciphera Pharmaceuticals, Eisai, EMD Serono, Exelixis, GlaxoSmithKline, Gilead, HiberCell, IQVA, Infinity, Ipsen, Jansen, Kanaph, Lilly, Merck, Nikang, Neomorph, Nuscan/PrecedeBio, Novartis, Oncohost, Pfizer, Roche, Sanofi/Aventis, Scholar Rock, Surface Oncology, Takeda, Tempest, Up-To-Date, CME events (Peerview, OncLive, MJH, CCO and others), outside the submitted work; Institutional patents filed on molecular alterations and immunotherapy response/toxicity, and ctDNA; Equity: Tempest, Pionyr, Osel, PrecedeBio, CureResponse, InnDura Therapeutics, Primium; Committees: NCCN, GU Steering Committee, ASCO (BOD 6-2024-, ESMO, ACCRU, KidneyCan; Medical writing and editorial assistance support may have been funded by Communications companies in part; No speaker’s bureau; Mentored several non-US citizens on research projects with potential funding (in part) from non-US sources/Foreign Components; The institution (Dana-Farber Cancer Institute) may have received additional independent funding of drug companies or/and royalties potentially involved in research around the subject matter.
Figures

References
-
- Lu, Q. et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature398, 723–728 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA101942/CA/NCI NIH HHS/United States
- R01 CA222355/CA/NCI NIH HHS/United States
- RO1 CA193675 and RO1 CA222355/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- 2P50CA101942-16 and 5P30CA006516-56/Dana-Farber/Harvard Cancer Center (DF/HCC)
- R01 CA193675/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

