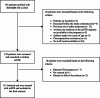
Evaluation of a modified short all oral treatment regimen for rifampicin-multidrug resistant tuberculosis in Dominican Republic
- PMID: 39924538
- PMCID: PMC11808990
- DOI: 10.1186/s12879-024-10417-w
Evaluation of a modified short all oral treatment regimen for rifampicin-multidrug resistant tuberculosis in Dominican Republic
Abstract
Background: This study aims to evaluate the effectiveness, safety, and impact on health-related quality of life (HQoL) of a fully oral shortened regimen for Rifampicin-Resistant/Multidrug-Resistant Tuberculosis (RR/MDR-TB) over 9 to 12 months under programmatic conditions.
Methods: A prospective cohort study was conducted on an all-oral modified Shortened Treatment Regimen (mSTR) comprising linezolid (Lzd), bedaquiline (Bdq), levofloxacin (Lfx), clofazimine (Cfz), and cycloserine (Cs). Patients with RR/MDR-TB were enrolled between January and December 2022 across seven drug-resistant TB units in the Dominican Republic.
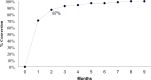
Results: A total of 113 patients were enrolled, with 87% achieving culture conversion at two months. Treatment outcomes revealed that 79% of patients were successfully treated and didn't relapse six months after the end of the treatment, 14% were lost to follow-up during the treatment, 6% deceased, and one experienced treatment failure due to Adverse Drug Reactions (ADRs). Adverse events of Special interest (AESI) were common, with 82% of patients experiencing at least one AE with high proportion of QT interval prolongation, elevated transaminases, and anemia. A total of 12% of the patients experiencing Serious Adverse Events (SAEs). Improvement in HQoL dimensions was noted throughout treatment, with the EQ-VAS score increasing by an average of 15.5 by treatment end.
Conclusion: The high treatment success rate of the 5-drug mSTR facilitated the adaptation and integration of a shortened treatment regimen lasting 9 to 12 months in routine care in Dominican Republic. SAEs were -rare. Although AESI were frequent, they were manageable in most cases. Continuous monitoring, particularly with regard to the use of Lzd and Bdq, is crucial to effectively mitigating risks. Since September 2023, this short all oral treatment regimen is the recommended approach for patients with RR/MDR-TB in the Dominican Republic.
Keywords: Bedaquiline; Dominican Republic; Health-Related Quality of Life; Linezolid; Multidrug-resistant; Safety; Short all-oral treatment; Treatment outcome; Tuberculosis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study obtained ethical approval from the WHO Research Ethics Review Board (Generic Protocol ID: ERC0003305, 08/06/2020) and the Ethics Committee of the Dr. Hugo Mendoza National Center for Research in Maternal and Child Health. Informed consent was obtained from each participant or respondent. For participants under the age of 16, consent to participate was obtained from their parents or legal guardians. All methods adhered to relevant guidelines and regulations. Consent for publication: All authors and respondents involved in this research have consented to the publication of all paper details, data, tables, and images. These materials will be freely accessible on the internet. Competing interests: The authors declare no competing interests. Disclaimer: The findings and conclusions of this article are the intellectual responsibility of its authors and do not necessarily represent the official position of Special Programme for Research and Training in Tropical Diseases of World health Organization, the Pan American Health Organization, Nacional Health Service, and Ministry of Public Health of Dominican Republic.
Figures
References
-
- Pan American Health Organization. Tuberculosis [Internet]. Pan American Health Organization. 2022 [cited 2023 Dec 23]. Available from: https://www.paho.org/en/topics/tuberculosis
-
- Melgen RE. Impacto De La Pandemia COVID-19 en El control De La Tuberculosis en República Dominicana. Ciencia Y Salud. 2022;6(3):95–103.
-
- World Health Organization. Global tuberculosis report 2023 [Internet]. Geneva; 2023 [cited 2023 Dec 22]. Available from: https://iris.who.int/
-
- Espinal MA, Báez J, Soriano G, Garcia V, Laszlo A, Reingold AL, et al. Drug-resistant tuberculosis in the Dominican Republic: results of a nationwide survey. Int J Tuberc Lung Dis. 1998;2(6):490–8. - PubMed
-
- Organización Panamericana de la Salud. Tuberculosis en las Américas. Informe regional 2021 [Internet]. Washington. 2022. Available from: https://iris.paho.org/bitstream/handle/10665.2/57084/9789275326497_spa.p...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources