Cutaneous Side Effects of PD-1 Inhibitors: A Single-Center Retrospective Study
- PMID: 39925013
- PMCID: PMC12082620
- DOI: 10.1111/ijd.17683
Cutaneous Side Effects of PD-1 Inhibitors: A Single-Center Retrospective Study
Abstract
Background: The introduction of immune checkpoint inhibitors (ICIs) opened a new era in cancer immunotherapy. In particular, PD-1 inhibitors have shown remarkable efficacy in various cancers, most notably melanoma. However, the widespread use of immune checkpoint inhibitors comes with the challenge of immune-related adverse events (irAEs), with cutaneous toxicities being the most prevalent.
Methods: A retrospective, single-center study was carried out to investigate the cutaneous side effects in patients diagnosed with melanoma and treated with PD-1 inhibitors (pembrolizumab or nivolumab) at the Department of Dermatology, Venereology, and Oncodermatology, University of Pécs, Hungary. The study included patients with stage III or IV melanoma who received PD-1 inhibitor monotherapy, either for metastatic or adjuvant purposes, from August 2015 to May 2022.
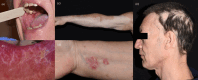
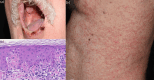
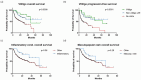
Results: A cohort of 174 patients was examined, with 29% experiencing cutaneous adverse events (cAEs). The most prevalent cutaneous toxicities were vitiligo (n = 18; 27%), maculopapular rash (n = 14; 21%), pruritus (n = 14; 21%), xerostomia (n = 8; 12%), and lichenoid dermatitis (n = 4; 6%). Treatment primarily involved topical corticosteroids and emollients, with a few cases requiring systemic therapy. Notably, the occurrence of dermatologic adverse events was associated with improved progression-free survival (PFS) (p = 0.007) and overall survival (OS) (p = 0.026) compared to those without any skin toxicity (p < 0.0001), emphasizing their potential prognostic significance. Our data were not influenced by any well-known prognostic factors of melanoma.
Conclusion: Our study contributes to the growing body of evidence supporting the prognostic value of cutaneous adverse events in patients treated with PD-1 inhibitors. Optimizing treatment strategies while maintaining oncologic therapy is essential, highlighting the role of dermatologists in multidisciplinary cancer care.
Keywords: PD‐1 inhibitor; immune checkpoint inhibitors; immune‐related cutaneous side effect; melanoma; skin toxicity.
© 2025 The Author(s). International Journal of Dermatology published by Wiley Periodicals LLC on behalf of the International Society of Dermatology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Freeman‐Keller M., Kim Y., Cronin H., Richards A., Gibney G., and Weber J. S., “Cancer Therapy: Clinical Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune‐Related Adverse Events and Association With Outcomes,” Clinical Cancer Research 22, no. 4 (2016): 886–894. - PMC - PubMed
-
- Bottlaender L., Amini‐Adle M., Maucort‐Boulch D., Robinson P., Thomas L., and Dalle S., “Cutaneous Adverse Events: A Predictor of Tumour Response Under Anti‐PD‐1 Therapy for Metastatic Melanoma, a Cohort Analysis of 189 Patients,” Journal of the European Academy of Dermatology and Venereology 34, no. 9 (2020): 2096–2105. - PubMed
-
- Sibaud V., “Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy,” American Journal of Clinical Dermatology 19, no. 3 (2018): 345–361. - PubMed
-
- Apalla Z. and Sibaud V., “Immunotherapy‐Mediated Dermatological Adverse Events: The Urgent Need for a Common, Clinically Meaningful, Management Strategy,” Support Care Cancer 28, no. 12 (2020): 5597–5599. - PubMed
MeSH terms
Substances
Grants and funding
- 1400-2-105-53366/Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences, Tehran, Iran
- Thematic Excellence Program 2021 Health Sub-programme of the Ministry for Innovation and Technology in Hungary, within the framework of the EGA-10 project of the University of Pécs
LinkOut - more resources
Full Text Sources
Medical

