Unlocking the secrets of the human gut microbiota: Comprehensive review on its role in different diseases
- PMID: 39926224
- PMCID: PMC11718612
- DOI: 10.3748/wjg.v31.i5.99913
Unlocking the secrets of the human gut microbiota: Comprehensive review on its role in different diseases
Abstract
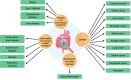
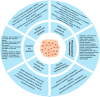
The human gut microbiota, a complex and diverse community of microorganisms, plays a crucial role in maintaining overall health by influencing various physiological processes, including digestion, immune function, and disease susceptibility. The balance between beneficial and harmful bacteria is essential for health, with dysbiosis - disruption of this balance - linked to numerous conditions such as metabolic disorders, autoimmune diseases, and cancers. This review highlights key genera such as Enterococcus, Ruminococcus, Bacteroides, Bifidobacterium, Escherichia coli, Akkermansia muciniphila, Firmicutes (including Clostridium and Lactobacillus), and Roseburia due to their well-established roles in immune regulation and metabolic processes, but other bacteria, including Clostridioides difficile, Salmonella, Helicobacter pylori, and Fusobacterium nucleatum, are also implicated in dysbiosis and various diseases. Pathogenic bacteria, including Escherichia coli and Bacteroides fragilis, contribute to inflammation and cancer progression by disrupting immune responses and damaging tissues. The potential for microbiota-based therapies, such as probiotics, prebiotics, fecal microbiota transplantation, and dietary interventions, to improve health outcomes is examined. Future research directions in the integration of multi-omics, the impact of diet and lifestyle on microbiota composition, and advancing microbiota engineering techniques are also discussed. Understanding the gut microbiota's role in health and disease is essential for formulating personalized, efficacious treatments and preventive strategies, thereby enhancing health outcomes and progressing microbiome research.
Keywords: Autoimmune disease; Cancer; Diabetics; Dysbiosis; Gut microbiota.
©The Author(s) 2025. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

