Novel immunochromatographic test for rapid detection of anti-factor H autoantibodies with an assessment of its clinical relevance
- PMID: 39926597
- PMCID: PMC11802491
- DOI: 10.3389/fimmu.2024.1527016
Novel immunochromatographic test for rapid detection of anti-factor H autoantibodies with an assessment of its clinical relevance
Abstract
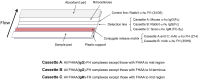
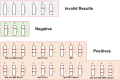
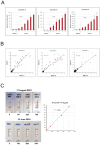
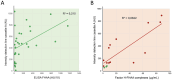
Factor H (FH) is a crucial complement regulator that prevents complement-mediated injury to healthy cells and tissues. This regulatory function can be disrupted by Factor H autoantibodies (FHAA), which then leads to diseases such as atypical hemolytic uremic syndrome (aHUS) and C3 Glomerulopathy (C3G). In pediatric aHUS, the FHAA incidence is ~10-15%, although in the Indian population, it rises to ~50%. The specific regions of FH targeted by FHAAs correlate with the pathogenic mechanism of the associated disease. In aHUS, FHAAs target the C-terminus, thereby impacting FH ability to recognize cell surfaces. In C3G, in contrast, FHAAs often target the N-terminus, generating an acquired functional FH deficiency. Detection and monitoring FHAAs are decisive for effectively treating patients. Current FHAA analysis normally identify free FHAAs that bind surface-bound FH using ELISA techniques. These methods require well-equipped laboratories and qualified staff, and do not measure FH-FHAA complexes, which can make it difficult to correlate titers with clinical outcomes. The visually-based immunochromatographic test (ICT) described herein allows for quick detection and quantification of IgG and IgM FH-FHAA complexes in human EDTA-plasma or serum. This ICT offers improved detection of FHAAs compared to ELISA as demonstrated by cases where the ICT identifies FH-FHAA complexes in samples that tested negative with the free FHAA ELISA. Importantly, the ICT indirectly informs on the amount of FH that is complexed with FHAAs, thus assessing the significance of the FHAA in disrupting the regulatory function of FH. Overall, this novel assay offers a simple, fast, cost-effective, and, likely, more clinically relevant alternative for diagnosing FHAAs in at-risk populations.
Keywords: C3-glomerulopathy; anti factor H autoantibodies; atypical hemolytic uremic syndrome; complement; diagnostic test; immunochromatographic test.
Copyright © 2025 Rodríguez de Córdoba, Reparaz, Sanchez, Pinto, Juana Lopez, Martin Merinero, Calvete, Perez-Perez, Jellison, Zhang, Smith, Moreno and Dominguez.
Conflict of interest statement
Authors IC and JP-P were employed in Secugen S. L. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

