Debut of enzyme-responsive anionic cyanine for overlap-free NIR-II-to-I dual-channel tumour imaging
- PMID: 39926710
- PMCID: PMC11801379
- DOI: 10.1039/d4sc06459c
Debut of enzyme-responsive anionic cyanine for overlap-free NIR-II-to-I dual-channel tumour imaging
Abstract
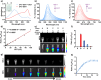
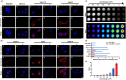
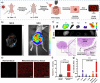
Bridging the disparity between traditional surgical resection imaging and ex vivo histopathology, fluorescence imaging is considered a promising tool in disease diagnosis and imaging navigation. Nevertheless, its usefulness is undermined by the variability of single-wavelength fluorescence signals and limited penetration of NIR-I (650-900 nm) bioimaging. In this work, we present a novel NIR-II ratiometric fluorescent probe (CFC-GSH) with γ-glutamyl transpeptidase (GGT) sensitivity for multifunctional bioimaging. This probe leverages a GSH-capped anionic cyanine, with advantages of high brightness, excellent photostability, high specificity and favourable biocompatibility. CFC-GSH exhibits an intrinsically stable NIR-II signal prior to triggering, which can be utilized for in vivo systemic circulation vessel outlining and microvascular imaging. At the tumour site with GGT over expression, an intramolecular S,N-rearrangement would initiate the conversion of sulphur-substituted cyanine to amino-substituted cyanine, resulting in a significant emission shift of 270 nm. Using the dual-channel signal changes, CFC-GSH effectively differentiates between subcutaneous hepatocellular carcinoma (HCC) and normal tissue and precisely localizes metastatic HCC tumours in the abdominal cavity. These results reveal that CFC-GSH exhibits promising potential as a multiprospective candidate tool for fluorescence screening and diagnostic imaging in various biological scenarios.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Lou H. Ji A. Qu C. Duan S. Liu H. Chen H. Cheng Z. Chem. Eng. J. 2022;449:137848–137856. doi: 10.1016/j.cej.2022.137848. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

