The use of newer anti-seizure medicines in women with epilepsy in pregnancy: A case series
- PMID: 39927179
- PMCID: PMC11804773
- DOI: 10.1016/j.ebr.2025.100741
The use of newer anti-seizure medicines in women with epilepsy in pregnancy: A case series
Abstract
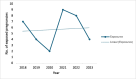
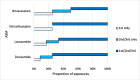
Epilepsy is a common serious neurological disorder, affecting approximately 28 per 10,000 pregnancies internationally each year. There are limited data on the use of newer anti-seizure medicines (ASMs) in pregnancy, despite increasing use. We aimed to describe the use of newer ASMs in women with epilepsy (WWE) attending the Rotunda Hospital, Dublin, in pregnancy, between 2018 and 2023. We conducted a retrospective case series using electronic health record data. All WWE with a medication order for a newer ASM and a completed pregnancy were included. We identified 34 pregnancies exposed to newer ASMs, namely zonisamide (35.2 %), brivaracetam (23.5 %), eslicarbazepine (23.5 %), lacosamide (17.6 %), and perampanel (2.9 %). Newer ASMs were used as monotherapy in 58.8 % cases. Levetiracetam was the most commonly prescribed concomitant ASM in polytherapy regimens (32.4 %). Seizures occurred during pregnancy or the postpartum period in 50.0 % and 14.7 % of pregnancies, respectively. Twenty-eight pregnancies (80 %) resulted in a livebirth, with median gestation and birth weight of 39 weeks' [IQR 2] and 3100 g [IQR 790]. One neonate exposed to polytherapy including eslicarbazepine was observed to have a minor anomaly at birth, not requiring follow-up. Findings show that in WWE, most pregnancies exposed to newer ASMs resulted in healthy livebirths at term without negative outcomes. A high proportion of polytherapy exposures and high rate of seizures during pregnancy suggests that this may be a cohort at greater risk for caesarean section or other complications. Findings should be interpreted with caution, with additional data needed to examine the impact of individual ASMs on outcomes.
Keywords: Brivaracetam; Eslicarbazepine; Lacosamide; Newer anti-seizure medicines; Pregnancy; Women with epilepsy; Zonisamide.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Knight M, Bunch K, Felker A, et al., editors. on behalf of MBRRACE-UK. Saving Lives, Improving Mothers’ Care Core Report - Lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2019-21. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2023.
-
- Medicines and Healthcare products. Antiepileptic drugs: Review of Safety of Use During Pregnancy. MHRA Public Assessment Report, January 2021. https://www.gov.uk/government/publications/public-assesment-report-of-an... (accessed 29 December 2024).
LinkOut - more resources
Full Text Sources
