Mapping cell diversity and dynamics in inflammatory temporomandibular joint osteoarthritis with pain at single-cell resolution
- PMID: 39927459
- PMCID: PMC11948589
- DOI: 10.1172/jci.insight.184379
Mapping cell diversity and dynamics in inflammatory temporomandibular joint osteoarthritis with pain at single-cell resolution
Abstract
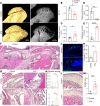
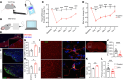
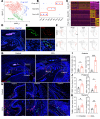
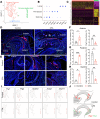
Temporomandibular joint (TMJ) osteoarthritis with pain is a highly prevalent disorder affecting patients' quality of life. A comprehensive understanding of cell type diversity and its dynamics in painful TMJ osteoarthritis (TMJOA) is lacking. Here, we utilized an inflammatory TMJOA mouse model via intra-articular injection of CFA. TMJOA mice exhibited cartilage remodeling, bone loss, synovitis, increased osteoarthritis (OA) score, and orofacial pain, recapitulating hallmark symptoms in patients. Single-cell transcriptomic profiling of the TMJ was performed in conjunction with mouse genetic labeling, tissue clearing, light sheet and confocal 3D imaging, multiplex RNAscope, and immunodetection. We visualized, reconstructed, and analyzed the distribution and density of nociceptive innervation of TMJ at single-axon levels. We systematically mapped the heterogeneity and anatomical position of blood endothelial cells, synovial fibroblasts, and immune cells, including Cx3cr1-positive barrier macrophages. Importantly, TMJOA mice exhibited enhanced neurovascular coupling, sublining fibroblast hyperplasia, inflammatory immune cell expansion, disrupted signaling-dependent cell-cell interaction, and a breakdown of the sandwich-like organization consisting of synovial barrier macrophages and fibroblasts. By utilizing a mouse model with combined TMJ pain history and OA, we reveal the cellular diversity, anatomical structure, and cell dynamics of the TMJ at single-cell resolution, which facilitate our understanding and potential targeting of TMJOA.
Keywords: Behavior; Bioinformatics; Cell biology; Inflammation; Osteoarthritis.
Figures




References
-
- Schiffman E, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD consortium network and orofacial pain special interest group†. J Oral Facial Pain Headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

