Prenatal alcohol exposure is associated with altered feto-placental blood flow and sex-specific placental changes
- PMID: 39927463
- PMCID: PMC11948586
- DOI: 10.1172/jci.insight.186096
Prenatal alcohol exposure is associated with altered feto-placental blood flow and sex-specific placental changes
Abstract
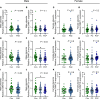
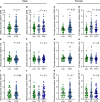
BACKGROUNDPrenatal alcohol exposure (PAE) around conception in preclinical models results in placental insufficiency, likely contributing to offspring abnormalities. Altered placental DNA methylation (DNAm) and gene expression suggest epigenetic mechanisms, perhaps involving impacts on methyl donor levels. PAE around conception in women is common but placental effects are rarely examined. This cohort study investigated associations between PAE around conception and intake/plasma measures of the methyl donors folate and choline, feto-placental blood flow, and placental growth measures, gene expression, and DNAm.METHODSPregnant participants delivered at Mater Mothers' Hospital, Brisbane, Queensland, Australia (n = 411). Dietary intake of choline and folate were calculated and plasma concentrations measured using mass spectrometry (MS) and clinical immunoanalyzer, respectively. Cerebroplacental ratio (CPR) was calculated using Doppler measurements. Placentas were weighed/measured at delivery and samples used to quantify methyl donors (MS), global DNAm (ELISA), and gene expression (quantitative PCR). Data were compared between control/abstinent and PAE groups, by fetal sex.RESULTSA CPR <5th-centile, indicating fetal brain sparing because of placental insufficiency, was found in 2% of controls and 18% of the PAE group, mostly male fetuses (63%). Compared with controls, male PAE placentas had reduced mean thickness and placental growth factor mRNA and DNAm, whereas female PAE placentas had increased S-adenosylmethionine and a trend toward increased DNAm.CONCLUSIONPAE around conception is associated with reduced CPR and altered placental growth measures, particularly in males, with potential implications for future health.FUNDINGNational Health and Medical Research Council (APP1191217) and Mary McConnel Career Boost Program for Women in Paediatric Research (WIS132020).
Keywords: Development; Growth factors; Molecular biology; Obstetrics/gynecology; Reproductive biology.
Conflict of interest statement
Figures



References
-
- Patra J, et al. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011;118(12):1411–1421. doi: 10.1111/j.1471-0528.2011.03050.x. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

