Hybrid Electro-optical Stimulation Improves Ischemic Brain Damage by Augmenting the Glymphatic System
- PMID: 39927473
- PMCID: PMC11967803
- DOI: 10.1002/advs.202417449
Hybrid Electro-optical Stimulation Improves Ischemic Brain Damage by Augmenting the Glymphatic System
Abstract
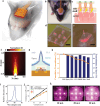
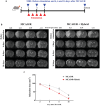
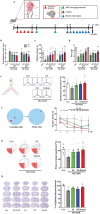
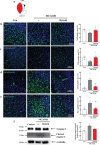
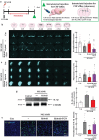
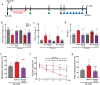
Ischemic brain injury not only results in significant neurological, motor, and cognitive impairment but also contributes to the accumulation of toxic solutes and proinflammatory cytokines in the infarction region, exacerbating ischemic brain damage. The glymphatic system, which is crucial for brain waste clearance and homeostasis, is impaired by ischemic injury, highlighting the importance of developing therapeutic strategies for poststroke complications. Herein, a novel hybrid electro-optical stimulation device is proposed that integrates near-infrared micro-light-emitting diode with transparent microneedles, enabling efficient noninvasive stimulation of the cortical area for ischemic stroke treatment. This study investigates whether this hybrid electro-optical stimulation enhances the glymphatic system function and ameliorates ischemic brain injury in the middle cerebral artery occlusion and reperfusion (MCAO/R) mice model. The results demonstrate that hybrid stimulation improves the neurological, motor, and cognitive functions and reduces brain atrophy following MCAO/R. Moreover, hybrid stimulation restores impaired glymphatic system function by modulation of aquaporin-4 (AQP4) polarization and alleviates the accumulation of proinflammatory cytokines such as IL-1β. Notably, AQP4 inhibition partly reverses the improved functional outcomes of hybrid stimulation. The findings suggest that targeting glymphatic drainage using hybrid electro-optical stimulation is a promising therapeutic approach for treating ischemic brain injury.
Keywords: aquaporin‐4 polarization; cerebrospinal fluid; glymphatic system; hybrid electro‐optical stimulation; ischemic stroke.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Martin S. S., Aday A. W., Almarzooq Z. I., Anderson C. A. M., Arora P., Avery C. L., Baker‐Smith C. M., Barone Gibbs B., Beaton A. Z., Boehme A. K., Commodore‐Mensah Y., Currie M. E., Elkind M. S. V., Evenson K. R., Generoso G., Heard D. G., Hiremath S., Johansen M. C., Kalani R., Kazi D. S., Ko D., Liu J., Magnani J. W., Michos E. D., Mussolino M. E., Navaneethan S. D., Parikh N. I., Perman S. M., Poudel R., Rezk‐Hanna M., et al., Circulation 2024, 149, e347. - PubMed
MeSH terms
Substances
Grants and funding
- RS-2021-NR056452/National Research Foundation of Korea (NRF), Korean government (MSIP)
- RS-2023-00217893/National Research Foundation of Korea (NRF), Korean government (MSIP)
- RS-2022-NR071112/Basic Science Research Program through the National Research Foundation of Korea (NRF), Ministry of Education
LinkOut - more resources
Full Text Sources
