Atrial cardiomyocyte-restricted cleavage of gasdermin D promotes atrial arrhythmogenesis
- PMID: 39927987
- PMCID: PMC11959185
- DOI: 10.1093/eurheartj/ehaf024
Atrial cardiomyocyte-restricted cleavage of gasdermin D promotes atrial arrhythmogenesis
Abstract
Background and aims: Enhanced inflammatory signalling causally contributes to atrial fibrillation (AF) development. Gasdermin D (GSDMD) is an important downstream effector of several inflammasome pathways. However, the role of GSDMD, particularly the cleaved N-terminal (NT)-GSDMD, in non-immune cells remains elusive. This study aimed to elucidate the function of NT-GSDMD in atrial cardiomyocytes (ACMs) and determine its contribution to atrial arrhythmogenesis.
Methods: Human atrial appendages were used to assess the protein levels and localization. A modified adeno-associated virus 9 was employed to establish ACM-restricted overexpression of NT-GSDMD in mice.
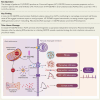
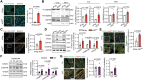
Results: The cleavage of GSDMD was enhanced in ACMs of AF patients. Atrial cardiomyocyte-restricted overexpression of NT-GSDMD in mice increased susceptibility to pacing-induced AF. The NT-GSDMD pore formation facilitated interleukin-1β secretion from ACMs, promoting macrophage infiltration, while up-regulating 'endosomal sorting complexes required for transport'-mediated membrane-repair mechanisms, which prevented inflammatory cell death (pyroptosis) in ACMs. Up-regulated NT-GSDMD directly targeted mitochondria, increasing mitochondrial reactive oxygen species (ROS) generation, which triggered proarrhythmic calcium-release events. The NT-GSDMD-induced arrhythmogenesis was mitigated by the mitochondrial-specific antioxidant MitoTEMPO. A mutant NT-GSDMD lacking pore-formation capability failed to cause mitochondrial dysfunction or induce atrial arrhythmia. Genetic ablation of Gsdmd prevented spontaneous AF development in a mouse model.
Conclusions: These findings establish a unique pyroptosis-independent role of NT-GSDMD in ACMs and arrhythmogenesis, which involves ROS-driven mitochondrial dysfunction. Mitochondrial-targeted therapy, either by reducing ROS production or inhibition of GSDMD, prevents AF inducibility, positioning GSDMD as a novel therapeutic target for AF prevention.
Keywords: Atrial fibrillation; ESCRT; Gasdermin D; Mitochondria.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

