SLPI controls neutrophil migration abilities and impacts neutrophil skin infiltration in experimental psoriasis
- PMID: 39928132
- PMCID: PMC11810868
- DOI: 10.1007/s00018-025-05606-y
SLPI controls neutrophil migration abilities and impacts neutrophil skin infiltration in experimental psoriasis
Abstract
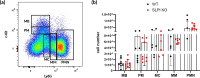
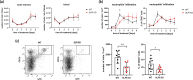
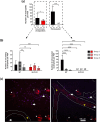
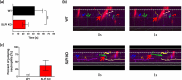
Skin infiltration by neutrophils is a hallmark of the chronic inflammatory skin disease psoriasis, yet the mechanisms underlying neutrophil recruitment and positioning in chronically inflamed skin remain poorly understood. In this study, we demonstrate the significant impact of a total genetic deficiency of secretory leukocyte protease inhibitor (SLPI) on neutrophil migration in mouse skin. Without SLPI, neutrophils displayed an unconventional migratory pattern, characterized by altered interactions with vessel walls and reduced efficiency in extravasating from blood vessels into skin tissue during the early stages of experimental psoriasis. This was associated with changes in tissue motility, positioning neutrophils farther from the skin entry vessels and closer to the skin surface. Neutrophil diapedesis was partially dependent on SLPI within the neutrophils themselves. The impact of SLPI on neutrophil movement was further supported by the increased migration of human neutrophils in the presence of neutrophil-penetrant recombinant SLPI. Additionally, our data suggest that neutrophils with varying capacities for vessel wall interaction are released from the bone marrow into circulation in an SLPI-dependent manner. These findings establish a role for SLPI in regulating the spatiotemporal infiltration of neutrophils into the skin in psoriasis, highlighting its relevance to psoriasis pathophysiology.
Keywords: Chronic inflammation; Dermis; Inhibitors of serine proteases; Innate immunity; Intravital microscopy; Neutrophil elastase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All animal procedures and experiments were performed in accordance with national and European legislation, following approval by the 2nd Local Institutional Animal Care and Use Committee (IACUC) in Krakow (# 298/2017 and 61/2023). All human studies were performed in accordance with guidelines established by the Jagiellonian University Institutional Bioethics Committee under approved protocols (#87/B/2014; 1072.6120.30.2020) and adhered to the Declaration of Helsinki. Human blood was collected from healthy individuals who were fully informed and had consented. Competing interests: The authors have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Christophers E, Metzler G, Rocken M (2014) Bimodal immune activation in psoriasis. Br J Dermatol 170(1):59–65 - PubMed
-
- Chen J, Zhu Z, Li Q, Lin Y, Dang E, Meng H, Sha N, Bai H, Wang G, An S, Shao S (2021) Neutrophils enhance cutaneous vascular dilation and permeability to aggravate psoriasis by releasing Matrix Metallopeptidase 9. J Invest Dermatol 141(4):787–799 - PubMed
-
- Henry CM, Sullivan GP, Clancy DM, Afonina IS, Kulms D, Martin SJ (2016) Neutrophil-derived proteases escalate inflammation through activation of IL-36 Family cytokines. Cell Rep 14(4):708–722 - PubMed
-
- Higashi Y, Yamakuchi M, Ibusuki A, Okubo A, Fukushige T, Hashiguchi T, Kanekura T (2024) Neutrophil-derived MicroRNA-1290 promotes keratinocyte proliferation in Psoriasis. J Invest Dermatol 144(7):1471–1478e6 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

