Response to Inhaled Nitric Oxide and Mortality Among Very Preterm Neonates With Pulmonary Hypertension
- PMID: 39928335
- PMCID: PMC11811801
- DOI: 10.1001/jamanetworkopen.2024.58843
Response to Inhaled Nitric Oxide and Mortality Among Very Preterm Neonates With Pulmonary Hypertension
Abstract
Importance: Clinical observations of immediate improvement in fraction of inspired oxygen (FiO2) in a proportion of cases is often cited as the rationale for using inhaled nitric oxide (iNO) in the management of acute pulmonary hypertension among very preterm neonates (gestational age, <32 weeks). However, the clinical effectiveness of such a response pattern remains underinvestigated.
Objective: To identify factors associated with predischarge mortality among very preterm neonates receiving iNO for acute pulmonary hypertension, with specific a priori emphasis on iNO responsiveness.
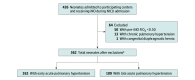
Design, setting, and participants: This prospective observational cohort study was conducted from January 1, 2018, to December 31, 2022, at 12 Canadian tertiary neonatal intensive care units. Consecutive very preterm neonates who received iNO for a diagnosis of acute pulmonary hypertension and pretreatment FiO2 of 0.50 or more were included. Neonates with congenital anomalies or those who were treated for chronic pulmonary hypertension with iNO were excluded. Early acute pulmonary hypertension (≤72 hours of age) and late acute pulmonary hypertension (>72 hours of age) cohorts were analyzed separately. Statistical analysis was performed from January 2023 to January 2024.
Exposure: Treatment with iNO for acute pulmonary hypertension.
Main outcomes and measures: The study cohorts were divided for comparison based on the primary outcome of predischarge mortality. Logistic regression analyses were used with predefined variables, including iNO responsiveness, to identify factors associated with mortality. A positive response to iNO was defined as a pre-iNO minus 4-hour post-iNO FiO2 of 0.20 or more.
Results: The early acute pulmonary hypertension group (mean [SD] birth gestational age, 26.3 [2.4] weeks; median treatment age, 1 day [IQR, 1-2 days]; 147 boys [56%]) included 262 neonates; 179 (68%) had a pre-iNO FiO2 of 1.0. The late acute pulmonary hypertension group (mean [SD] birth gestational age, 24.9 [1.7] weeks; median treatment age, 13 days [IQR, 9-20 days]; 72 boys [66%]) included 109 neonates; 51 (47%) had a pre-iNO FiO2 of 1.0. Neonates with early acute pulmonary hypertension more frequently had a positive iNO response (71% [186 of 262] vs 41% [45 of 109]) and lower mortality (34% [90 of 262] vs 49% [53 of 109]) than those with late acute pulmonary hypertension. Accounting for pretreatment illness factors, greater reduction in FiO2 with iNO remained associated with lower mortality for neonates with early acute pulmonary hypertension (adjusted odds ratio per FiO2 reduction of 0.10, 0.74 [95% CI, 0.65-0.84]). For those with late acute pulmonary hypertension, however, only pretreatment illness severity (lower pre-iNO FiO2 and higher pre-iNO pH), and not positive response to iNO (adjusted odds ratio, 0.47 [95% CI, 0.17-1.30]), was associated with mortality.
Conclusions and relevance: In this cohort study of very preterm neonates with acute pulmonary hypertension treated with iNO, responsiveness to iNO was associated with improved outcomes during the first 72 hours of age. The prognostic role of iNO response in acute pulmonary hypertension presenting after 72 hours of age remains unclear. Future studies should investigate the distinct pathophysiological mechanisms associated with late acute pulmonary hypertension in this population.
Conflict of interest statement
Figures

References
-
- Abman SH, Hansmann G, Archer SL, et al. ; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society . Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037-2099. doi:10.1161/CIR.0000000000000329 - DOI - PubMed
-
- Askie LM, Ballard RA, Cutter GR, et al. ; Meta-analysis of Preterm Patients on Inhaled Nitric Oxide Collaboration . Inhaled nitric oxide in preterm infants: an individual-patient data meta-analysis of randomized trials. Pediatrics. 2011;128(4):729-739. doi:10.1542/peds.2010-2725 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

