Astrocytic EphA4 signaling is important for the elimination of excitatory synapses in Alzheimer's disease
- PMID: 39928878
- PMCID: PMC11848297
- DOI: 10.1073/pnas.2420324122
Astrocytic EphA4 signaling is important for the elimination of excitatory synapses in Alzheimer's disease
Abstract
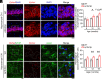
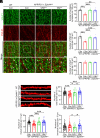
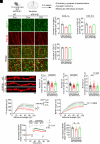
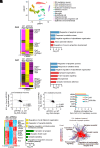
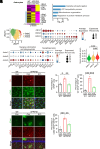
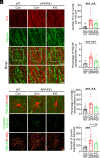
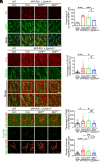
Cell surface receptors, including erythropoietin-producing hepatocellular A4 (EphA4), are important in regulating hippocampal synapse loss, which is the key driver of memory decline in Alzheimer's disease (AD). However, the cell-specific roles and mechanisms of EphA4 are unclear. Here, we show that EphA4 expression is elevated in hippocampal CA1 astrocytes in AD conditions. Specific knockout of astrocytic EphA4 ameliorates excitatory synapse loss in the hippocampus in AD transgenic mouse models. Single-nucleus RNA sequencing analysis revealed that EphA4 inhibition specifically decreases a reactive astrocyte subpopulation with enriched complement signaling, which is associated with synapse elimination by astrocytes in AD. Importantly, astrocytic EphA4 knockout in an AD transgenic mouse model decreases complement tagging on excitatory synapses and excitatory synapses within astrocytes. These findings suggest an important role of EphA4 in the astrocyte-mediated elimination of excitatory synapses in AD and highlight the crucial role of astrocytes in hippocampal synapse maintenance in AD.
Keywords: Alzheimer’s disease; Eph receptors; complement signaling; reactive astrocytes; synapse loss.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
References
MeSH terms
Substances
Grants and funding
- 2021YFE0203000/the National Key R&D Program of China grant
- the Collaborative Research Fund [C6027-19GF]/the Research Grants Council of Hong Kong
- the Theme-Based Research Scheme [T13-605/18 W]/the Research Grants Council of Hong Kong
- the General Research Fund [HKUST16102019]/the Research Grants Council of Hong Kong
- the Areas of Excellence Scheme of the University Grants Committee (AoE/M-604/16)/the Research Grants Council of Hong Kong
LinkOut - more resources
Full Text Sources
Miscellaneous

