Validity, Accuracy, and Safety Assessment of an Aerobic Interval Training Using an App-Based Prehabilitation Program (PROTEGO MAXIMA Trial) Before Major Surgery: Prospective, Interventional Pilot Study
- PMID: 39928941
- PMCID: PMC11851035
- DOI: 10.2196/55298
Validity, Accuracy, and Safety Assessment of an Aerobic Interval Training Using an App-Based Prehabilitation Program (PROTEGO MAXIMA Trial) Before Major Surgery: Prospective, Interventional Pilot Study
Abstract
Background: Major surgery is associated with significant morbidity and a reduced quality of life, particularly among older adults and individuals with frailty and impaired functional capacity. Multimodal prehabilitation can enhance functional recovery after surgery and reduce postoperative complications. Digital prehabilitation has the potential to be a resource-sparing and patient-empowering tool that improves patients' preoperative status; however, little remains known regarding their safety and accuracy as medical devices.
Objective: This study aims to test the accuracy and validity of a new software in comparison to the gold-standard electrocardiogram (ECG)-based heart rate measurement.
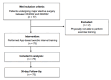
Methods: The PROTEGO MAXIMA trial was a prospective interventional pilot trial assessing the validity, accuracy, and safety of an app-based exercise program. The Prehab App calculates a personalized, risk-stratified aerobic interval training plan based on individual risk factors and utilizes wearables to monitor heart rate. Healthy students and patients undergoing major surgery were enrolled. A structured risk assessment was conducted, followed by a 6-minute walking test and a 37-minute supervised interval session. During the exercise, patients wore app-linked wearables for heart rate and distance measurements, which were compared with standard ECG and treadmill measurements. Safety, accuracy, and usability assessments included testing alarm signals, while the occurrence of adverse events served as the primary and secondary outcome measures.
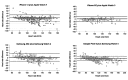
Results: A total of 75 participants were included. The mean heart rate differences between wearables and standard ECG were ≤5 bpm (beats per minute) with a mean absolute percentage error of ≤5%. Regression analysis revealed a significant impact of the BMI (odds ratio 0.90, 95% CI 0.82-0.98, P=.02) and Timed Up and Go Test score (odds ratio 0.12, 95% CI 0.03-0.55, P=.006) on the accuracy of heart rate measurement; 29 (39%) patients experienced adverse events: pain (5/12, 42%), ECG electrode-related skin irritations (2/42, 17%), dizziness (2/42, 17%), shortness of breath (2/42, 17%), and fatigue (1/42, 8%). No cardiovascular or serious adverse events were reported, and no serious device deficiency was detected. There were no indications of clinically meaningful overexertion based on laboratory values measured before and after the 6-minute walking test and exercise. The differences in means and ranges were as follows: lactate (mmol/l), mean 0.04 (range -3 to 6; P=.47); creatinine kinase (U/l), mean 12 (range -7 to 43; P<.001); and sodium (mmol/l), mean -2 (range -11 to 12; P<.001).
Conclusions: The interventional trial demonstrated the high safety of the exercise program and the accuracy of heart rate measurements using commercial wearables in patients before major surgery, paving the way for potential remote implementation in the future.
Trial registration: German Clinical Trials Register DRKS00026985; https://drks.de/search/en/trial/DRKS00026985 and European Database on Medical Devices (EUDAMED) CIV-21-07-0307311.
International registered report identifier (irrid): RR2-10.1136/bmjopen-2022-069394.
Keywords: accuracy; aerobic; aerobic training; app; digital health; heart rate; major surgery; management; medical device; oncology; pilot study; prehab; prehabilitation; quality of life; safety and quality; safety management; smartwatches; surgery; surgical; surgical oncology; validity; wearable; wearables.
©Sara Fatima Faqar Uz Zaman, Svenja Sliwinski, Lisa Mohr-Wetzel, Julia Dreilich, Natalie Filmann, Charlotte Detemble, Dora Zmuc, Felix Chun, Wojciech Derwich, Waldemar Schreiner, Wolf Bechstein, Johannes Fleckenstein, Andreas A Schnitzbauer. Originally published in JMIR mHealth and uHealth (https://mhealth.jmir.org), 10.02.2025.
Conflict of interest statement
Conflicts of Interest: AAS, DZ, and CD are founders of Capreolos GmbH, a spin-off from Goethe University established to set up the regulatory framework for developing a medical device for prehabilitation. JF became a shareholder of Capreolos after the termination of the trial. All other authors declare that they have no direct or indirect conflicts of interest.
Figures

References
-
- Nepogodiev D, Martin J, Biccard B, Makupe A, Bhangu A, Nepogodiev D, Martin J, Biccard B, Makupe A, Ademuyiwa A, Adisa AO, Aguilera M, Chakrabortee S, Fitzgerald JE, Ghosh D, Glasbey JC, Harrison EM, Ingabire JA, Salem H, Lapitan MC, Lawani I, Lissauer D, Magill L, Moore R, Osei-Bordom DC, Pinkney TD, Qureshi AU, Ramos-De la Medina A, Rayne S, Sundar S, Tabiri S, Verjee A, Yepez R, Garden OJ, Lilford R, Brocklehurst P, Morton DG, Bhangu A. Global burden of postoperative death. The Lancet. 2019 Feb;393(10170):401. doi: 10.1016/s0140-6736(18)33139-8. - DOI - PubMed
-
- World Health Organization (WHO) WHO Guidelines for Safe Surgery: Safe Surgery Saves Lives. Geneva, Switzerland: World Health Organization; 2009.
-
- Fuertes-Guiró Fernando, Viteri Velasco E. The impact of frailty on the economic evaluation of geriatric surgery: hospital costs and opportunity costs based on meta-analysis. J Med Econ. 2020 Aug 20;23(8):819–830. doi: 10.1080/13696998.2020.1764965. https://www.tandfonline.com/doi/10.1080/13696998.2020.1764965?url_ver=Z3... - DOI - DOI - PubMed
-
- Barberan-Garcia A, Ubre M, Pascual-Argente N, Risco R, Faner J, Balust J, Lacy A, Puig-Junoy J, Roca J, Martinez-Palli G. Post-discharge impact and cost-consequence analysis of prehabilitation in high-risk patients undergoing major abdominal surgery: secondary results from a randomised controlled trial. Br J Anaesth. 2019 Oct;123(4):450–456. doi: 10.1016/j.bja.2019.05.032. https://linkinghub.elsevier.com/retrieve/pii/S0007-0912(19)30436-2 S0007-0912(19)30436-2 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

