Single-cell dynamics of breakthrough toxicities after anakinra prophylaxis for axicabtagene ciloleucel in lymphoma
- PMID: 39928957
- PMCID: PMC12051123
- DOI: 10.1182/bloodadvances.2024015161
Single-cell dynamics of breakthrough toxicities after anakinra prophylaxis for axicabtagene ciloleucel in lymphoma
Abstract
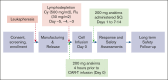
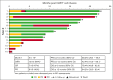
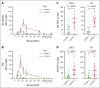
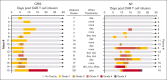
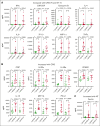
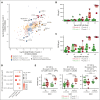
Chimeric antigen receptor (CAR) T-cell (CAR-T) therapy is limited by cytokine release syndrome (CRS) and neurotoxicity (NT). We sought to use once-daily prophylactic anakinra, an interleukin-1 (IL-1) receptor antagonist, to prevent CRS/NT that would require hospitalization (grade ≥2) in patients receiving axicabtagene ciloleucel for large-cell lymphoma, with the goal of facilitating outpatient therapy and management. Our study, in line with others, demonstrates that once-daily prophylactic anakinra is insufficient to prevent the development of toxicities that would require hospitalization in most patients. As part of the initial study design, we prospectively incorporated single-cell RNA sequencing to gain insight into the molecular immune signaling associated with breakthrough CRS and NT despite anakinra prophylaxis. In patients who developed breakthrough CRS or NT, we found that interferon gamma (IFN-γ) pathways and ligand-receptor activities were significantly enriched, as were cytokine levels of IFN-γ and CXCL10 in CD14+ monocytes. This correlated with increased IFN-γ and other cytokines in the peripheral blood. In infused CAR-T products, IL-4 and IL-10 anti-inflammatory pathways were negatively associated with grade ≥2 toxicities, regardless of anakinra treatment. These data identify IFN-γ as a potential key mechanism in CAR-T-associated toxicities, which is not inhibited by anakinra but may be otherwise targetable. This trial was registered at www.ClinicalTrials.gov as #NCT04150913.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.J.F. receives research funding from Kite and Arcellx; and receives consulting fees from Sobi, Novartis, Bristol Myers Squibb (BMS), Kite/Gilead, Iovance, and Johnson & Johnson/Legend. C.A.J. reports receiving consulting fees from Kite/Gilead, Novartis, BMS/Celgene, ImmPACT Bio, Caribou Bio, Instil Bio, Miltenyi, Ipsen, Morphosys, ADC Therapeutics, AbbVie, AstraZeneca, Sana, Synthekine; and reports research funding from Kite/Gilead and Pfizer. Y.-B.C. consults for Incyte, Takeda, Novartis, Novo Nordisk, Editas, Pharmacosmos, and Vor. Z.D. receives research support from Incyte Corp, REGiMMUNE Corp, and Taiho Oncology, Inc; and has received consulting fees from Sanofi, Incyte Corp, MorphoSys AG, Inhibrx, PharmaBiome AG, and ONO PHARMA. A.R.E.-J. consults for GlaxoSmithKline, Novartis, Incyte, AIM Pathway, and Tuesday Health. P.C.J. has served as a consultant for Seagen, ADC Therapeutics, AbbVie, Incyte, and AstraZeneca; and reports research funding from AstraZeneca. R.W.M. served on the advisory boards for Genmab, Adaptive Biotechnologies, BMS, AbbVie, Inteillia, Epizyme, and Seattle Genetics; consults for Alphasights; and has received institutional research funding from Merck, BMS, Genmab, and Genentech/Roche. Authors R.S., S.F., and J.N.Kite report employment with Kite, a Gilead company, and stock or other ownership in Gilead Sciences. G.G. receives research funds from IBM, Pharmacyclics, and Ultima Genomics; is an inventor on patent applications related to MSMuTect, MSMutSig, MSIDetect, POLYSOLVER, SignatureAnalyzer-GPU, and MinimuMM-seq; is a founder, consultant, and holds privately held equity in Scorpion Therapeutics; is a founder and holds privately help equity in Predicta Biosciences; and received travel support from Caris Life Sciences. M.V.M. is an inventor on patents related to adoptive cell therapies, held by Massachusetts General Hospital and the University of Pennsylvania (some licensed to Novartis); holds equity in 2seventybio, Genocea, Oncternal, and Neximmune; serves on the board of directors of 2seventybio; and has served as a consultant for multiple companies involved in cell therapies (M.V.M.’s interests were reviewed and are managed by Massachusetts General Hospital, and Mass General Brigham in accordance with their conflict-of-interest policies). The remaining authors declare no competing financial interests.
Figures


References
-
- Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. - PubMed
-
- Jacobson CA, Hunter B, Armand P, et al. Axicabtagene ciloleucel in the real world: outcomes and predictors of response, resistance and toxicity [abstract] Blood. 2018;132(suppl 1) Abstract 92.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

