Trajectory analysis of hepatic stellate cell differentiation reveals metabolic regulation of cell commitment and fibrosis
- PMID: 39929812
- PMCID: PMC11811062
- DOI: 10.1038/s41467-025-56024-4
Trajectory analysis of hepatic stellate cell differentiation reveals metabolic regulation of cell commitment and fibrosis
Abstract
Defining the trajectory of cells during differentiation and disease is key for uncovering the mechanisms driving cell fate and identity. However, trajectories of human cells remain largely unexplored due to the challenges of studying them with human samples. In this study, we investigate the proteome trajectory of iPSCs differentiation to hepatic stellate cells (diHSCs) and identify RORA as a key transcription factor governing the metabolic reprogramming of HSCs necessary for diHSCs' commitment, identity, and activation. Using RORA deficient iPSCs and pharmacologic interventions, we show that RORA is required for early differentiation and prevents diHSCs activation by reducing the high energetic state of the cells. While RORA knockout mice have enhanced fibrosis, RORA agonists rescue multi-organ fibrosis in in vivo models. Notably, RORA expression correlates negatively with liver fibrosis and HSCs activation markers in patients with liver disease. This study reveals that RORA regulates cell metabolic plasticity, important for mesoderm differentiation, pericyte quiescence, and fibrosis, influencing cell commitment and disease.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.C. and P.S-B. have a patent (EP2016/079464) regarding the hepatic stellate cell differentiation. The remaining authors declare no competing interests.
Figures


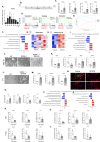
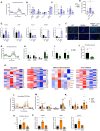
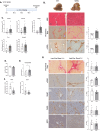
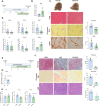
References
-
- Zhao, X., Kwan, J. Y., Yip, K., Liu, P. P. & Liu, F.-F. Targeting metabolic dysregulation for fibrosis therapy. Nat. Rev. Drug Discov.19, 57–75 (2019). - PubMed
-
- Taylor, R. S. et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology158, 1611–1625.e12 (2020). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

