Uncovering protein glycosylation dynamics and heterogeneity using deep quantitative glycoprofiling (DQGlyco)
- PMID: 39930009
- PMCID: PMC12170336
- DOI: 10.1038/s41594-025-01485-w
Uncovering protein glycosylation dynamics and heterogeneity using deep quantitative glycoprofiling (DQGlyco)
Abstract
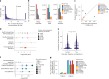
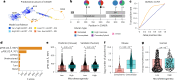
Protein glycosylation regulates essential cellular processes such as signaling, adhesion and cell-cell interactions; however, dysregulated glycosylation is associated with diseases such as cancer. Here we introduce deep quantitative glycoprofiling (DQGlyco), a robust method that integrates high-throughput sample preparation, highly sensitive detection and precise multiplexed quantification to investigate protein glycosylation dynamics at an unprecedented depth. Using DQGlyco, we profiled the mouse brain glycoproteome, identifying 177,198 unique N-glycopeptides-25 times more than previous studies. We quantified glycopeptide changes in human cells treated with a fucosylation inhibitor and characterized surface-exposed glycoforms. Furthermore, we analyzed tissue-specific glycosylation patterns in mice and demonstrated that a defined gut microbiota substantially remodels the mouse brain glycoproteome, shedding light on the link between the gut microbiome and brain protein functions. Additionally, we developed a novel strategy to evaluate glycoform solubility, offering new insights into their biophysical properties. Overall, the in-depth profiling offered by DQGlyco uncovered extensive complexity in glycosylation regulation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

