Multifunctional hyaluronic acid-based biomimetic/pH-responsive hybrid nanostructured lipid carriers for treating bacterial sepsis
- PMID: 39930418
- PMCID: PMC11812216
- DOI: 10.1186/s12929-024-01114-6
Multifunctional hyaluronic acid-based biomimetic/pH-responsive hybrid nanostructured lipid carriers for treating bacterial sepsis
Abstract
Introduction: The application of biomimetic and stimuli-responsive nanocarriers displays considerable promise in improving the management of bacterial sepsis and overcoming antimicrobial resistance. Therefore, the study aimed to synthesize a novel hyaluronic acid-lysine conjugate (HA-Lys) and to utilize the attributes of the synthesized HA-Lys with Tocopherol succinate (TS) and Oleylamine (OLA) in the formulation of multifunctional biomimetic pH-responsive HNLCs loaded with vancomycin (VCM-HNLCs), to combat bacterial sepsis.
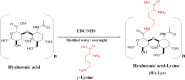
Methods: A novel hyaluronic acid-lysine conjugate (HA-Lys) was synthesized and characterized using FTIR and 1H NMR spectroscopy. Vancomycin-loaded hybrid nanosystems (VCM-HNLCs) were prepared through hot homogenization ultrasonication and evaluated for particle size, polydispersity index (PDI), zeta potential (ZP), and encapsulation efficiency (EE%). In vitro biocompatibility was assessed via MTT assay and red blood cell hemolysis test. The binding affinity to TLR2 and TLR4 was measured using microscale thermophoresis (MST). Drug release was evaluated using the dialysis bag method. Antimicrobial activity against MRSA and efflux pump inhibition were also determined. Efficacy was demonstrated in an MRSA-induced sepsis mice model.
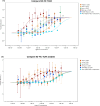
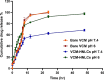
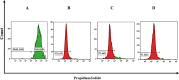
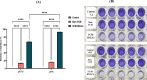
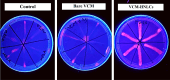
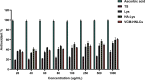
Results: The VCM-HNLCs, produced via hot homogenization ultrasonication, exhibited particle size (PS), polydispersity index (PDI), zeta potential (ZP), and encapsulation efficiency (EE%) of 110.77 ± 1.692 nm, 0.113 ± 0.022, - 2.92 ± 0.210 mV, and 76.27 ± 1.200%, respectively. In vitro, biocompatibility was proven by hemolysis and cytotoxicity studies. The VCM-HNLCs demonstrated targetability to human Toll-like receptors (TLR 2 and 4) as validated by microscale thermophoresis (MST). VCM-HNLCs showed a twofold reduction in MIC values at physiological pH compared to the bare VCM against S. aureus and MRSA for 48 h. While at pH 6.0, MIC values were reduced by fourfold in the first 24 h and by eightfold in the subsequent 48 and 72 h against tested strains. Furthermore, VCM-HNLCs showed inhibitory effects against MRSA efflux pumps, reactive oxygen species (ROS), and lipopolysaccharide (LPS)-induced hyperinflammation. In an MRSA-induced sepsis mice model, VCM-HNLCs demonstrated superior efficacy compared to free VCM, significantly eliminated bacteria and improved survival rates.
Conclusions: Overall, these results highlight the potential of VCM-HNLCs as novel multifunctional nanocarriers to combat antimicrobial resistance (AMR) and enhance sepsis outcomes.
Keywords: Antibiotic delivery; Bacterial sepsis; Biomimetic; Hyaluronic acid-lysine; Hybrid nanocarriers; PH-responsive.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animal studies were carried out with approval from the University of Calgary Animal Care Committee and complied with the Canadian Council for Animal Care Guidelines. Consent for publication: Not applicable. Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
-
- WHO. Global report on the epidemiology and burden of sepsis. 2020 9 September 2020; https://www.who.int/publications/i/item/9789240010789. Accessed 5 May 2023.
-
- WHO. Global antimicrobial resistance and use surveillance system (GLASS) report: 2022. 2022 09 December 2022; https://www.who.int/news/item/09-12-2022-report-signals-increasing-resis.... Accessed 30 Jan 2023.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

