Outcomes of the ROSE Sustainment (ROSES) Study, a sequential multiple assignment randomized implementation trial to determine the minimum necessary intervention to sustain a postpartum depression prevention program in agencies serving low-income pregnant people
- PMID: 39930503
- PMCID: PMC11812186
- DOI: 10.1186/s13012-025-01420-z
Outcomes of the ROSE Sustainment (ROSES) Study, a sequential multiple assignment randomized implementation trial to determine the minimum necessary intervention to sustain a postpartum depression prevention program in agencies serving low-income pregnant people
Abstract
Background: This Sequential Multiple Assignment Randomized Trial (SMART) was conducted to determine minimum implementation support needed for agencies serving pregnant people on public assistance to adopt and sustain the ROSE (Reach Out, Stay Strong, Essentials for mothers of newborns) postpartum depression (PPD) prevention program.
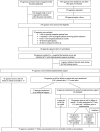
Methods: Enrolled prenatal agencies (N=98) received thorough initial implementation support (initial training + written sustainment planning). Agencies were identified as at risk for non-sustainment within the first 15 months (N=56) were randomized to: (1) no additional implementation support (N=12), or (2) quarterly implementation support (coaching and feedback; N=44). If agencies receiving quarterly implementation supports were still at risk and within the first 15 months (N=29), they were randomized to: (1) continued quarterly support (N=14), or (2) monthly implementation support (N=15). No implementation support occurred after 18 months. Follow-ups occurred quarterly and then at 18, 24, and 30 months. Outcomes included sustainment of core program elements, agency PPD rates, reach, and costs/cost-effectiveness of each sustainment step.
Results: Twice as many agencies as expected (41 of 98; 42%) delivered ROSE with fidelity for 15+ months after receiving thorough initial implementation support only. For agencies at risk for non-sustainment, no effects of adding quarterly implementation supports were observed. However, adding monthly supports (versus quarterly) for agencies still at risk resulted in higher monthly percent of core ROSE elements sustained and more months ROSE was sustained with fidelity with large (Cohen's d = 0.73 and 0.80) effect sizes, and improved reach over 30 months. Many agencies did not consistently collect PPD rates, making results difficult to interpret. Mean implementation costs (including implementation support and agency staff time) per agency were $1,849 (SD $1,429) for agencies receiving initial implementation support only, $2,699 (SD $1,837) for those receiving initial and quarterly implementation support, and $4,059 (SD $1,763) for those receiving initial, quarterly, and ultimately monthly implementation support.
Conclusions: The cost of agency-wide ROSE implementation is far less than the cost of a single untreated case of PPD ($33,484). We suggest implementing ROSE through thorough training and written sustainment planning. For agencies not sustaining, adding monthly support can promote sustainment and improve reach.
Trial registration: Registered June 14, 2018 at clinicaltrials.gov, NCT03267563 ( https://clinicaltrials.gov/study/NCT03267563 ).
Keywords: Cost-effectiveness; Implementation; Postpartum depression; Prenatal care; Prevention; Public assistance; Sustainment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The protocol for this study was approved by the Michigan State University Biomedical and Health Institutional Review Board (protocol IP# 00056524). Consent for participation in the study will be obtained using an electronic informed consent form. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
Protocol for the ROSE sustainment (ROSES) study, a sequential multiple assignment randomized trial to determine the minimum necessary intervention to maintain a postpartum depression prevention program in prenatal clinics serving low-income women.Implement Sci. 2018 Aug 22;13(1):115. doi: 10.1186/s13012-018-0807-9. Implement Sci. 2018. PMID: 30134941 Free PMC article. Clinical Trial.
-
Education for contraceptive use by women after childbirth.Cochrane Database Syst Rev. 2012 Aug 15;(8):CD001863. doi: 10.1002/14651858.CD001863.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Jul 29;(7):CD001863. doi: 10.1002/14651858.CD001863.pub4. PMID: 22895923 Updated.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2011 Jul 6;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. Cochrane Database Syst Rev. 2011. PMID: 21735394
-
Incentives for increasing prenatal care use by women in order to improve maternal and neonatal outcomes.Cochrane Database Syst Rev. 2015 Dec 15;2015(12):CD009916. doi: 10.1002/14651858.CD009916.pub2. Cochrane Database Syst Rev. 2015. PMID: 26671418 Free PMC article.
-
Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003510. doi: 10.1002/14651858.CD003510.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2011 Jul 06;(7):CD003510. doi: 10.1002/14651858.CD003510.pub3. PMID: 17253490 Updated.
Cited by
-
Implementation science aims to improve healthcare by validating effective interventions.Proc Natl Acad Sci U S A. 2025 Jul 29;122(30):e2517704122. doi: 10.1073/pnas.2517704122. Epub 2025 Jul 23. Proc Natl Acad Sci U S A. 2025. PMID: 40699924 Free PMC article. No abstract available.
References
-
- Scheirer M. Is sustainability possible? A review and commentary on empirical studies of program sustainability. Am J Eval. 2005;23:320–47.
-
- Shediac-Rizkallah M, Bone LR. Planning for the sustainability of community-based health programs: Conceptual frameworks and future directions for research, practice and policy. Health Educ Res. 1998;13(1):87–108. - PubMed
-
- Johnson JE, Wiltsey-Stirman S, Sikorskii A, Miller T, King A, Blume JL, Pham X, Moore Simas TA, Poleshuck E, Weinberg R, Zlotnick C. Protocol for the ROSE sustainment (ROSES) study, a sequential multiple assignment randomized trial to determine the minimum necessary intervention to maintain a postpartum depression prevention program in prenatal clinics serving low-income women. Implement Sci. 2018;13(1):115. - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

