Assessment the real-world safety of intravitreal dexamethasone implant (Ozurdex): novel insights from a comprehensive pharmacovigilance analysis utilizing the FAERS database
- PMID: 39930531
- PMCID: PMC11809023
- DOI: 10.1186/s40360-025-00866-7
Assessment the real-world safety of intravitreal dexamethasone implant (Ozurdex): novel insights from a comprehensive pharmacovigilance analysis utilizing the FAERS database
Abstract
Objective: The intravitreal dexamethasone implant (Dex) is widely used for various ocular conditions, including diabetic macular edema (DME), retinal vein occlusion (RVO), and non-infectious uveitis. Despite its efficacy, concerns remain regarding its safety profile. This study aims to analyze the adverse events (AEs) associated with Dex reported in the FDA Adverse Event Reporting System (FAERS) database from 2010 to 2024.
Methods: Data were extracted from FAERS, focusing on cases where Dex was the primary suspect drug. The dataset was processed to eliminate duplicates and incomplete entries. Disproportionality analysis, including Reporting Odds Ratio (ROR) and Proportional Reporting Ratio (PRR), was used to detect safety signals. AEs were categorized by system organ class (SOC) and preferred term (PT).
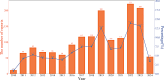
Results: A total of 1,588 adverse event reports (AERs) were analyzed, revealing a significant upward trend. The Eye disorders was the most commonly reported SOC, with strong disproportionality signals (ROR: 45.11; PRR: 23.71). Key AEs identified at the PT level included Corneal decompensation, Choroidal hematoma, and Posterior capsule rupture, which were not listed on the drug label. Considering the reported numbers, the Endophthalmitis was the most common AE. Additionally, a significant proportion of AEs were observed within the first seven days post-administration, emphasizing the need for monitoring.
Conclusion: While Dex remains an effective treatment option for ocular conditions, its use is associated with significant risks, particularly regarding unexpected and severe complications such as corneal decompensation. Continuous pharmacovigilance and detailed patient monitoring are essential to mitigate these risks. Future studies should focus on prospective designs and comprehensive clinical data to better understand the safety profile of Dex.
Keywords: Adverse events; Disproportionality analysis; FAERS; Intravitreal dexamethasone implant; Pharmacovigilance.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Investig Ophthalmol Vis Sci. 2011;52:80–6. - PubMed
-
- Rosenblatt A, Udaondo P, Cunha-Vaz J, Sivaprasad S, Bandello F, Lanzetta P, et al. A collaborative retrospective study on the efficacy and Safety of Intravitreal Dexamethasone Implant (Ozurdex) in patients with Diabetic Macular Edema: the European DME Registry Study. Ophthalmology. 2020;127:377–93. - PubMed
-
- Haller JA, Bandello F, Belfort R Jr., Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–e11461133. - PubMed
-
- Pohlmann D, Vom Brocke GA, Winterhalter S, Steurer T, Thees S, Pleyer U. Dexamethasone inserts in noninfectious uveitis: a single-center experience. Ophthalmology. 2018;125:1088–99. - PubMed
-
- Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical