Impact of nebulizers on nanoparticles-based gene delivery efficiency: in vitro and in vivo comparison of jet and mesh nebulizers using branched-polyethyleneimine
- PMID: 39930696
- PMCID: PMC11816613
- DOI: 10.1080/10717544.2025.2463428
Impact of nebulizers on nanoparticles-based gene delivery efficiency: in vitro and in vivo comparison of jet and mesh nebulizers using branched-polyethyleneimine
Abstract
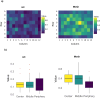
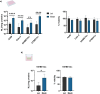
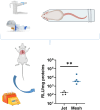
Nanoparticles-based gene delivery has emerged as a promising approach for the treatment of genetic diseases based on efficient delivery systems for therapeutic nucleic acids (NAs) into the target cells. For pulmonary diseases such as cystic fibrosis (CF), chronic obstructive pulmonary diseases (COPD), infectious disease or lung cancer, aerosol delivery is the best choice to locally deliver NAs into the lungs. It is, therefore, important to investigate the effects of nebulization conditions on the efficiency of delivery. To this purpose, the non-viral vector branched polyethyleneimine (b-PEI, 25 kDa) was investigated for plasmid delivery by aerosol. Two types of nebulizers, jet nebulizer and mesh nebulizer, were compared regarding the properties of the nanoparticles (NPs) formed, the efficiency of NAs delivery in vitro and in vivo models and the pulmonary deposition. The results indicate that the mesh nebulizer has a better gene delivery performance than the jet nebulizer in this application. This superiority was demonstrated in terms of size, concentration, distribution of NPs and efficiency of NAs delivery. However, pulmonary deposition appears to be similar regardless of the nebulizer used, and the difference between the two systems lies in the inhalable dose. These results underline the crucial role of nebulization techniques in optimizing aerosol-mediated gene delivery by b-PEI and highlight the potential of mesh nebulizers as promising tools to improved gene therapy. Therefore, the comparison must be performed for each gene therapy formulation to determine the most suitable nebulizer.
Keywords: Gene delivery; aerosol; branched-polyethyleneimine; cystic fibrosis; jet nebulizer; lung; mesh nebulizer; nebulization.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Ari A. May (2014). Jet, ultrasonic, and mesh nebulizers: an evaluation of nebulizers for better clinical outcomes. Eurasian J Pulmonol 16:1–7. doi:10.5152/ejp.2014.00087. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
