Pharmacokinetic profile and in vivo anticancer efficacy of anagrelide administered subcutaneously in rodents
- PMID: 39930717
- PMCID: PMC11816618
- DOI: 10.1080/10717544.2025.2463433
Pharmacokinetic profile and in vivo anticancer efficacy of anagrelide administered subcutaneously in rodents
Abstract
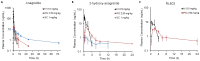
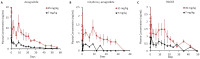
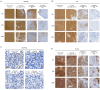
Anagrelide (ANA) is a phosphodiesterase 3A (PDE3A) inhibitor, commonly prescribed for essential thrombocythemia. It also functions as a molecular glue, inducing complex formation between PDE3A and Schlafen 12. This association either triggers apoptosis or inhibits proliferation in tumor cells, supporting its use in cancer therapy. Conventionally administered orally, ANA undergoes rapid metabolism and elimination, resulting in a short drug exposure time at the site of action. Here, we explored the pharmacokinetic profile of a subcutaneously (SC) injected ANA formulation in Sprague-Dawley rats by quantifying plasma ANA and metabolite concentrations using liquid-chromatography-tandem mass spectrometry. We further evaluated the in vivo tumor regression efficacy of orally and SC administered ANA in a patient-derived gastrointestinal stromal xenograft mouse model - UZLX-GIST2B - characterized by a KIT exon 9 driver mutation. The SC ANA exhibited extended-release plasma concentration-time profiles compared to intravenous and oral administrations. After a single administration in rats, plasma concentrations of ANA were detected up to 56 days later, and ANA metabolites up to 30 days later. The SC formulation also significantly reduced tumor volumes and demonstrated dose-dependent histological responses, nearly eradicating tumor tissue in 11 days with the highest dose. These findings suggest that the SC slow-release formulation maintains stable drug concentrations during treatment, potentially improving therapeutic efficacy at the target site.
Keywords: Anagrelide; GIST; pharmacokinetics; slow-release; subcutaneous.
Conflict of interest statement
Harri Sihto owns stocks of Sartar Therapeutics Ltd. Mikael Maksimow and Maria Lahtinen are employed by Sartar Therapeutics Ltd. The other authors declare no conflict of interest. The costs of the animal studies were sponsored by Sartar Therapeutics Ltd.
Figures

References
-
- Alexander-Curtis M, Duan J, Seeklander A, et al. (2017). Safety, tolerability and pharmacokinetic profile of recombinant human tissue kallikrein, DM199, after intravenous and subcutaneous administration in healthy volunteers. Int J Clin Trials 4:139–46. doi: 10.18203/2349-3259.ijct20174861. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
