Health-related Quality of Life and hospital costs of Finnish melanoma patients participating in the second Multicenter Selective Lymphadenectomy Trial (MSLT-II)
- PMID: 39930784
- PMCID: PMC11833325
- DOI: 10.2340/1651-226X.2025.42314
Health-related Quality of Life and hospital costs of Finnish melanoma patients participating in the second Multicenter Selective Lymphadenectomy Trial (MSLT-II)
Abstract
Background and purpose: After reports that complete lymph node dissection (CLND) did not improve melanoma-specific survival of sentinel lymph node (SLN)-positive patients, the use of CLND has diminished but it is still carried out for selected patients. We sought to assess differences in Health-Related Quality of Life (HRQoL) and tertiary care costs among the Finnish Multicenter Selective Lymphadenectomy Trial (MSLT)-II-patients.
Patients/materials and methods: A total of 52 patients randomized to CLND and 55 to nodal observation completed a modified version of the standardized and validated, RAND-36 questionnaire at baseline, 4 months and annually up to 5 years. Tertiary care costs between the groups were also compared.
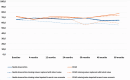
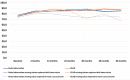
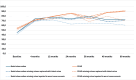
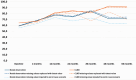
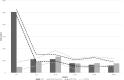
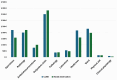
Results: At 60 months, the mean HRQoL score for the CLND and observation groups for General Health were 77.3 versus 65.0 (p = 0.007, adjusted p = 0.065), for role limitations due to physical health 89.5 versus 72.3 (p = 0.029, adjusted p = 0.203) and for role limitations due to emotional problems 91.4 versus 71.9 (p = 0.006, adjusted p = 0.065) and at 48 months, 92.8 versus 71.3 (p = 0.002, adjusted p = 0.056). Median costs per patient were higher in the CLND group at 4 months but the difference disappeared during follow-up.
Interpretation: This study suggests that undergoing CLND after a positive SLN biopsy is not a predictor of worse HRQoL. CLND generates greater costs initially, but there seem to be no major differences in total cost per patient between the two groups.
Conflict of interest statement
M.B.F. has the following conflicts of interest to disclose: Advisory Boards: Bristol Myers Squbb, Merck, Novartis, Instil Bio, Regeneron, Delcath Systems. J.F.T. has received honoraria for advisory board participation from BMS Australia, MSD Australia, GSK and Provectus Biopharmaceuticals, and conference and travel support from GSK and Provectus Biopharmaceuticals.
Figures
References
-
- Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–67. 10.1016/S1470-2045(16)00141-8 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

