Liposomal encapsulation of cholecalciferol mitigates in vivo toxicity and delays tumor growth
- PMID: 39931063
- PMCID: PMC11807986
- DOI: 10.3389/fimmu.2025.1529007
Liposomal encapsulation of cholecalciferol mitigates in vivo toxicity and delays tumor growth
Abstract
Introduction: Vitamin D3 (cholecalciferol) has demonstrated potential anticancer properties, but its clinical application is limited by associated toxicity at effective doses. This study investigated the use of liposomal encapsulation to increase the therapeutic efficacy of vitamin D3 while mitigating its toxicity.
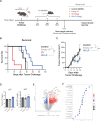
Methods: Liposomal vitamin D3 (VD-LP) was prepared via the film-hydration method and characterized for particle size, polydispersity index, encapsulation efficiency, and long-term stability. In vitro gene expression modulation was evaluated in monocytic THP-1 cells, and antiproliferative effects were assessed in HT29 (colorectal), BT474 (breast), and TRAMP-C1 (prostate) cancer cell lines. In vivo antitumor efficacy and toxicity were tested in a mouse model with subcutaneously implanted MC38 tumors. Tumor growth, survival rates, and serum calcium and phosphate levels were analyzed.
Results: VD-LP demonstrated high encapsulation efficiency and stability over 90 days, with a consistent particle size of approximately 83 nm. VD-LP modulated immune-related and metabolic gene expression in THP-1 cells, including upregulation of antimicrobial peptides and vitamin D receptor genes. VD-LP showed superior antiproliferative effects compared to free vitamin D3 in all tested cancer cell lines. In vivo, VD-LP delayed tumor growth and improved survival without causing hypercalcemia, highlighting its favorable toxicity profile.
Discussion: Liposomal encapsulation of vitamin D3 significantly improves its anticancer efficacy while mitigating toxicity, making it a promising strategy for future cancer therapies. VD-LP shows potential for enhanced therapeutic applications with reduced adverse effects, warranting further clinical exploration.
Keywords: anticancer efficacy; gene expression; liposomal encapsulation; tumor growth; vitamin D3.
Copyright © 2025 Ezcurra-Hualde, Zalba, Bella, Arrizabalaga, Risson, García-Fuentes, Gomar, Ardaiz, Belsue, Ruiz-Guillamon, Serrano-Alcaide, Salgado, Aranda, Garrido and Berraondo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. PB declared that he was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

