Anakinra for dengue patients with hyperinflammation: protocol for a randomized double-blind placebo-controlled trial
- PMID: 39931105
- PMCID: PMC11809184
- DOI: 10.12688/wellcomeopenres.21017.1
Anakinra for dengue patients with hyperinflammation: protocol for a randomized double-blind placebo-controlled trial
Abstract
Background: Novel host-directed therapies are urgently needed for patients with dengue, particularly those at high risk of developing severe disease. Broad immunosuppression using corticosteroids in unselected patients with dengue has so far been unsuccessful. Patients with hyperinflammation (raised CRP and/or ferritin levels) are at highest risk of poor outcomes in dengue. Anakinra is a licensed, bio-engineered form of the naturally occurring IL-1R antagonist which has shown efficacy in other acute viral-associated hyperinflammatory syndromes.
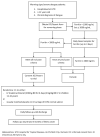
Methods: This is a randomized placebo-controlled phase II trial of anakinra in 160 patients ≥ 12 years old, diagnosed as having dengue with warning signs or severe dengue and the hyperinflammatory syndrome (plasma ferritin >2000 ng/ml). Participants will receive a 4-day course of either anakinra or placebo. The primary endpoint is the efficacy of anakinra measured by the delta mSOFA score* (change in mSOFA score over 4 days after randomization). The accompanying immunological and transcriptomic analyses aim to identify novel mechanisms and pathways that may represent future biomarkers and therapeutic targets.
Discussion: The observed immunomodulatory benefit of anakinra in acute viral-associated hyperinflammatory syndromes including COVID-19 and auto-immune diseases makes this medication a promising potential treatment for dengue patients with hyperinflammation. This trial will assess the safety and efficacy of anakinra in patients with severe dengue or at high risk of developing life-threatening dengue disease.
Registration: ClinicalTrials.gov (NCT05611710).
Keywords: IL-1 blockade; IL-1 receptor antagonist; anakinra; dengue; dengue with warning signs; interleukin-1 blockade; severe dengue; trial.
Plain language summary
Dengue is a globally important viral infection spread by mosquitoes. Most patients recover after a week, however a minority can progress to severe dengue with shock (a collapse of the circulatory system) or severe organ damage, which can be life-threatening. There are currently no specific therapies to change the disease course or outcomes. New treatments are urgently needed, especially for those with the most severe form of the disease. Research has shown that patients who have an excessive inflammatory response—when the body’s immune response to the virus is unregulated—have worse outcomes. Modulating this immune response could be a potential treatment approach. Recent studies suggested that interleukin-1 (IL-1) may play an important role in driving the hyper-inflammation during dengue. Blocking IL-1 could regulate the body’s response and improve clinical outcomes. In this clinical trial, we aim to investigate whether IL-1 blockade - using an established drug, anakinra, is safe and effective to treat moderate-to-severe dengue patients. Anakinra has already demonstrated safety and efficacy in hyperinflammatory syndromes caused by other viral infections, such as COVID-19. The study will recruit 160 patients, hospitalised with moderate to severe dengue who exhibit an excessive inflammatory response (indicated by a high level of the inflammatory marker, ferritin). Participants will receive either a 4-day course of anakinra or a placebo (visually matched syringes containing normal saline). Participants will be monitored closely, including their clinical manifestations, laboratory tests, and changes in disease severity over the 4-day treatment period, to assess the treatment's safety and effectiveness. Additionally, we aim to explore immunological and gene expression changes to identify new potential biomarkers and treatments. In summary, anakinra has demonstrated benefits in regulating immune responses in other hyperinflammatory conditions related to acute viral infections. This study will evaluate the safety and efficacy of anakinra in treating patients with moderate-severe dengue and hyperinflammation.
Copyright: © 2024 Huyen TB et al.
Conflict of interest statement
No competing interests were disclosed.
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous