RET inhibition overcomes resistance to combined CDK4/6 inhibitor and endocrine therapy in ER+ breast cancer
- PMID: 39931212
- PMCID: PMC11808005
- DOI: 10.3389/fonc.2024.1497093
RET inhibition overcomes resistance to combined CDK4/6 inhibitor and endocrine therapy in ER+ breast cancer
Abstract
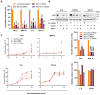
Background: Combined CDK4/6 inhibitor (CDK4/6i) and endocrine therapy significantly improve the outcome of patients with advanced estrogen receptor-positive (ER+) breast cancer. However, resistance to this treatment and disease progression remains a major clinical challenge. High expression of the receptor tyrosine kinase REarranged during Transfection (RET) has been associated with resistance to endocrine therapy in breast cancer, but the role of RET in CDK4/6i treatment response/resistance remains unexplored.
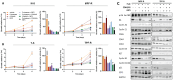
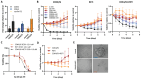
Methods: To identify gene expression alterations associated with resistance to combined endocrine therapy and CDK4/6i, we performed RNA sequencing of two ER+ breast cancer cell models resistant to this combined therapy. The functional role of RET was assessed by siRNA-mediated RET silencing and targeted inhibition with the FDA/EMA-approved RET-selective inhibitor selpercatinib in resistant breast cancer cells and patient-derived organoids (PDOs). RET silencing was evaluated mechanistically using global gene expression and pathway analysis. The clinical relevance of RET expression in ER+ breast cancer was investigated by gene array analysis of primary tumors treated with endocrine therapy and by immunohistochemical scoring of metastatic lesions from patients who received combined CDK4/6i and endocrine therapy.
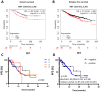
Results: We show that RET is upregulated in ER+ breast cancer cell lines resistant to combined CDK4/6i and fulvestrant compared to isogenic cells resistant to fulvestrant alone. siRNA-mediated silence of RET in high RET-expressing, combined CDK4/6i- and fulvestrant-resistant cells reduced their growth partially by affecting cell cycle regulators of the G2-M phase and E2F targets. Notably, targeting RET with selpercatinib in combination with CDK4/6i inhibited the growth of CDK4/6i-resistant cell lines and resensitized ER+ breast cancer patient-derived organoids resistant to CDK4/6i. Finally, analysis of RET expression in ER+ breast cancer patients treated with endocrine therapy showed that high RET expression correlated with poor clinical outcomes. We further observed a shorter median survival to combined CDK4/6i and endocrine therapy in patients with RET-positive compared to RET-negative tumors, but this difference did not reach statistical significance.
Conclusions: Our findings show that RET is overexpressed in ER+ metastatic breast cancer resistant to combined CDK4/6i and endocrine therapy, rendering RET inhibition a promising therapeutic approach for patients who experience disease progression on combined CDK4/6i and endocrine therapy.
Keywords: CDK4/6 inhibitor; RET; drug resistance; estrogen receptor-positive breast cancer; selpercatinib.
Copyright © 2025 Kindt, Ehmsen, Traynor, Policastro, Nissen, Jakobsen, Hundebøl, Johansen, Bak, Arbajian, Staaf, Ditzel and Alves.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. . PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro . Breast Cancer Res. (2009) 11:R77. doi: 10.1186/bcr2419 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

