Umbilical Cord Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Modulate Skin Matrix Synthesis and Pigmentation
- PMID: 39931529
- PMCID: PMC11807784
- DOI: 10.2147/IJN.S497940
Umbilical Cord Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Modulate Skin Matrix Synthesis and Pigmentation
Abstract
Introduction: Research has unveiled the remarkable properties of extracellular vesicles derived from mesenchymal stromal cells (MSCs), particularly in promoting wound healing, aiding re-epithelialization, revitalizing aging skin, and inhibiting hyperpigmentation. However, investigations into the potential of small extracellular vesicles from umbilical cord-derived MSCs (UC-MSC-sEVs) in reducing scarring and preventing hyperpigmentation remain limited. Therefore, this study aims to evaluate the impact of UC-MSC-sEVs on the synthesis of the skin's extracellular matrix (ECM) and pigmentation using in vitro models.
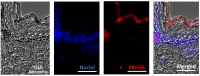
Methods: The study investigated the impact of characterized UC-MSC-sEVs on various aspects including the proliferation, migration, antioxidant activity, and ECM gene expression of human dermal fibroblasts (HDF). Additionally, the effects of UC-MSC-sEVs on the proliferation, melanin content, and tyrosinase (TYR) activity of human melanoma cells (MNT-1) were examined. Furthermore, ex vivo models were employed to evaluate the skin permeation of PKH26-labelled UC-MSC-sEVs.
Results: The findings indicated that a high concentration of UC-MSC-sEVs positively influenced the proliferation of HDF. However, no changes in cell migration rate were observed. While the expressions of collagen type 1 and type 3 remained unaffected by UC-MSC-sEVs treatment, there were dose-dependent increases in the gene expressions of fibronectin, matrix metallopeptidase (MMP) 1, and MMP 3. Furthermore, UC-MSC-sEVs treatment did not impact the antioxidative superoxide dismutase (SOD) expression in HDF. Although UC-MSC-sEVs did not alter the proliferation of MNT-1 cells, it did result in a dose-dependent reduction in melanin synthesis without affecting TYR activity. However, when it was applied topically, UC-MSC-sEVs failed to penetrate the skin barrier and remained localized within the stratum corneum layer even after 18 hours.
Conclusion: These results highlight the potential of UC-MSC-sEVs in stimulating HDF proliferation, regulating ECM synthesis, and reducing melanin production. This demonstrates the promising application of UC-MSC-sEVs in medical aesthetics for benefits such as scar reduction, skin rejuvenation, and skin lightening.
Keywords: anti-scarring; extracellular vesicles; medical aesthetic; mesenchymal stromal cell; pigmentation.
© 2025 Kee et al.
Conflict of interest statement
Ms Li Ting Kee reports a patent PI2024006022 pending to. Dr Min Hwei Ng reports a patent WJMSC exosomes for skin aesthetic use pending to None; and A company, Ming Medical Sdn Bhd, sponsors the study. However, academic researchers have developed the protocol for the derivation of the exosome entirely. Dr Jia Xian Law reports a patent An anti-scarring and anti-pigmentation composition pending to Universiti Kebangaan Malaysia and Ming Medical Sdn Bhd. The authors report no other conflicts of interest in this work.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

