A framework for modelling whole-lung and regional transfer factor of the lung for carbon monoxide using hyperpolarised xenon-129 lung magnetic resonance imaging
- PMID: 39931664
- PMCID: PMC11808933
- DOI: 10.1183/23120541.00442-2024
A framework for modelling whole-lung and regional transfer factor of the lung for carbon monoxide using hyperpolarised xenon-129 lung magnetic resonance imaging
Abstract
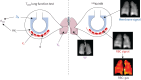
Background: Pulmonary gas exchange is assessed by the transfer factor of the lungs (T L) for carbon monoxide (T LCO), and can also be measured with inhaled xenon-129 (129Xe) magnetic resonance imaging (MRI). A model has been proposed to estimate T L from 129Xe MRI metrics, but this approach has not been fully validated and does not utilise the spatial information provided by three-dimensional 129Xe MRI.
Methods: Three models for predicting T L from 129Xe MRI metrics were compared: 1) a previously-published physiology-based model, 2) multivariable linear regression and 3) random forest regression. Models were trained on data from 150 patients with asthma and/or COPD. The random forest model was applied voxel-wise to 129Xe images to yield regional T L maps.
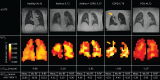
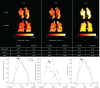
Results: Coefficients of the physiological model were found to differ from previously reported values. All models had good prediction accuracy with small mean absolute error (MAE): 1) 1.24±0.15 mmol·min-1·kPa-1, 2) 1.01±0.06 mmol·min-1·kPa-1, 3) 0.995±0.129 mmol·min-1·kPa-1. The random forest model performed well when applied to a validation group of post-COVID-19 patients and healthy volunteers (MAE=0.840 mmol·min-1·kPa-1), suggesting good generalisability. The feasibility of producing regional maps of predicted T L was demonstrated and the whole-lung sum of the T L maps agreed with measured T LCO (MAE=1.18 mmol·min-1·kPa-1).
Conclusion: The best prediction of T LCO from 129Xe MRI metrics was with a random forest regression framework. Applying this model on a voxel-wise level to create parametric T L maps provides a useful tool for regional visualisation and clinical interpretation of 129Xe gas exchange MRI.
Copyright ©The authors 2025.
Conflict of interest statement
Conflicts of interest: J.H. Pilgrim-Morris has no conflicts of interest to declare. L.J. Smith is a co-investigator on investigator-lead research grants from The Cystic Fibrosis Trust, Vertex Pharmaceuticals and The Sheffield Children's Hospital Charity, and has received support from AstraZeneca to attend research meetings. H. Marshall is a co-investigator on investigator-lead research grants of GlaxoSmithKline and the Engineering and Physical Sciences Research Council, and has received support from AstraZeneca to attend research meetings. B.A. Tahir has no conflicts of interest to declare. G.J. Collier has no conflicts of interest to declare. N.J. Stewart has no conflicts of interest to declare. J.M. Wild has received investigator led grants from AstraZeneca, GlaxoSmithKline, Vertex and GE Healthcare, has received consulting fees from GE Healthcare and consultancy fees from Vertex Ltd for speaking at image advisory meetings for lung MRI, and received support from AstraZeneca to attend the 2021 European Respiratory Society meeting.
Figures



References
-
- Modi P, Cascella M. Diffusing Capacity of the Lungs for Carbon Monoxide. Treasure Island (FL), StatPearls Publishing, 2023.
LinkOut - more resources
Full Text Sources
Miscellaneous
