Pharmacological Strategies for Providing Patients With Delirium Relief From Terminal Dyspnea: A Secondary Data Analysis
- PMID: 39931840
- PMCID: PMC11811727
- DOI: 10.1002/cam4.70677
Pharmacological Strategies for Providing Patients With Delirium Relief From Terminal Dyspnea: A Secondary Data Analysis
Abstract
Introduction: Systemic opioids are recommended as a pharmacological treatment for dyspnea, and antipsychotics are widely used for delirium. Because little is known about optimal palliative pharmacological strategies for dyspnea in patients with delirium, this study explored the symptom course in such cases, including the use of opioids and antipsychotics.
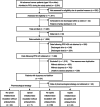
Methods: This was a secondary analysis of a multicenter prospective and observational study. We consecutively enrolled adult patients with advanced cancer at palliative care units in Japan. The eligibility criteria for their participation were a dyspnea Integrated Palliative care Outcome Scale (IPOS) score ≥ 2 and the presence of delirium. We investigated pharmacological strategies, IPOS for dyspnea, and delirium symptoms using item 9 of the Memorial Delirium Assessment Scale.
Results: Of the 1896 patients, 141 were found eligible and were analyzed. Eighty-two (58%) patients had agitated delirium, and the median survival period was 4 days. Regarding pharmacological strategy, 31 (22%) received opioid initiation or dose escalation, whereas 92 (65%) used regular antipsychotics. Although mean dyspnea IPOS scores significantly decreased from Day 1 to Day 2 (0.44, 95% CI: 0.24-0.64), the proportion of responders (IPOS score ≤ 1) was 21% (30/141). In the agitated delirium group, the proportion of remaining agitation symptoms at Day 2 was 74% (61/82).
Conclusions: The combined distressing symptoms of dyspnea and delirium during the last days of life are likely to be refractory suffering, which shows a poor response to pharmacological interventions, including opioids and antipsychotics.
Keywords: antipsychotic agents; delirium; dyspnea; neoplasm; opioid; palliative care.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

