Activation of CXCR3+ Tfh cells and B cells in lymph nodes during acute HIV-1 infection correlates with HIV-specific antibody development
- PMID: 39932316
- PMCID: PMC11915809
- DOI: 10.1128/jvi.01532-24
Activation of CXCR3+ Tfh cells and B cells in lymph nodes during acute HIV-1 infection correlates with HIV-specific antibody development
Abstract
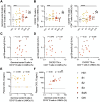
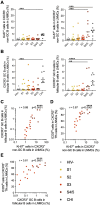
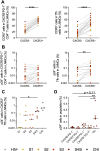
Lymph node T follicular helper (Tfh) cells and germinal center (GC) B cells are critical to generate potent antibodies but are rarely possible to study in humans. To understand how Tfh/GC B-cell interactions during acute HIV-1 infection (AHI) impact the generation of HIV-specific antibodies, we performed a unique cross-sectional analysis of inguinal lymph node biopsies taken prior to antiretroviral therapy (ART) initiation in AHI. Although total Tfh and GC B cell frequencies did not change during AHI, increased frequencies of proliferating Th1-like CXCR3+ Tfh, CXCR3+ non-GC B cells, and total CXCR3+ GC B cells correlated with gp120-specific IgG antibody levels in AHI. Frequencies of proliferating CXCR3+ Tfh in AHI also correlated with gp120-specific IgG antibody levels after 48 weeks of ART, antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and increased antibody binding to infected cells after ART. Importantly, while beneficial for antibody development, CXCR3+ Tfh cells were also infected by HIV-1 at higher frequencies than their CXCR3- counterparts and may contribute to the initial dissemination of HIV-1 in follicles. Together, these data suggest that activation of CXCR3+ Tfh cells is associated with induction of the germinal center response and subsequent antibody development, making these cells an important target for future therapeutic interventions.
Importance: Early initiation of antiretroviral therapy (ART) is important to limit the seeding of the long-lasting HIV-1 reservoir; however, it also precludes the development of HIV-specific antibodies that can help control the virus if ART is stopped. Antibody development occurs within germinal centers in the lymph node and requires activation of both antigen-specific B cells and T follicular helper cells (Tfh), a specialized CD4+ cell that provides B cell help. To understand how early ART initiation may prohibit antibody development, we analyzed the frequencies and activation status of Tfh and B cells in lymph node biopsies collected in the different stages of acute HIV-1 infection. Our data suggest that decreased antibody development after early ART initiation may be due to limited germinal center development at the time of treatment and that new interventions that target activation of CXCR3+ Tfh may be beneficial to increase long-term HIV-specific antibody levels.
Keywords: B-cell responses; Tfh cells; human immunodeficiency virus; humoral immunity; lymph node.
Conflict of interest statement
J.A. has received honoraria from Merck, ViiV Healthcare, Roche, AbbVie, and Gilead for her participation in advisory meetings. The other authors declare no conflicts of interest.
Figures



References
-
- Gray ES, Madiga MC, Moore PL, Mlisana K, Abdool Karim SS, Binley JM, Shaw GM, Mascola JR, Morris L. 2009. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J Virol 83:11265–11274. doi:10.1128/JVI.01359-09 - DOI - PMC - PubMed
-
- Doria-Rose NA, Klein RM, Manion MM, O’Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol 83:188–199. doi:10.1128/JVI.01583-08 - DOI - PMC - PubMed
-
- Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, et al. . 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol 83:7337–7348. doi:10.1128/JVI.00110-09 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

