Relationship of OCT-Based Diabetic Retinal Neurodegeneration to the Development and Progression of Diabetic Retinopathy: A Cohort Study
- PMID: 39932471
- PMCID: PMC11817850
- DOI: 10.1167/iovs.66.2.32
Relationship of OCT-Based Diabetic Retinal Neurodegeneration to the Development and Progression of Diabetic Retinopathy: A Cohort Study
Abstract
Purpose: To evaluate the relationship between diabetic retinal neurodegeneration (DRN), as quantified by optical coherence tomography (OCT), to the development of diabetic retinopathy (DR), progression of DR, and development of proliferative DR (PDR).
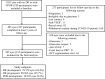
Methods: This was a prospective cohort study, including 385 eyes with no DR or nonproliferative DR at baseline. The thicknesses of the macular ganglion cell-inner plexiform layer (m-GCIPL), macular retinal nerve fiber layer, and peripapillary RNFL (p-RNFL) were measured using Cirrus OCT (Carl Zeiss Meditec, Dublin, CA, USA). DR outcomes were determined from macula- and optic disc-centered fundus photographs, following the modified Airlie House classification system. Cox proportional hazards models were used to estimate hazard ratio (HR) adjusting for age, mean arterial blood pressure, diabetes mellitus duration, HbA1c, diabetic kidney disease, axial length, OCT signal strength, and disc area (for p-RNFL only).
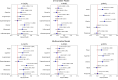
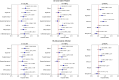
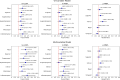
Results: After a median follow-up of 6.2 years (range 5.0-7.7 years), 79 eyes developed DR, 99 eyes developed DR progression, and 38 eyes developed PDR. Thinner mean and sectorial m-GCIPL thicknesses were significantly associated with higher risk of DR development, with HRs ≥ 1.373 (1.023-1.843), except for the superonasal and superotemporal sectors. Similar to DR development, thinner m-GCIPL thicknesses were significantly associated with DR progression and PDR development, with HRs ranging from 1.306 (1.094-1.559) to 2.331 (1.524-3.566). Additionally, the inclusion of inferior m-GCIPL thickness significantly improved the predictive discrimination for DR development (C statistics: 0.661 vs. 0.705, P < 0.001), and DR progression (C statistics: 0.704 vs. 0.729, P < 0.001), as well as inferotemporal m-GCIPL for PDR development (C statistic: 0.917 vs. 0.930, P < 0.001) beyond established risk factors.
Conclusions: OCT measurements that elucidate DRN may enhance prognostic identification and predictive discrimination of DR development, DR progression, and PDR development beyond established risk factors.
Conflict of interest statement
Disclosure:
Figures
References
-
- Antonetti DA, Klein R, Gardner TW.. Diabetic retinopathy. N Engl J Med. 2012; 366: 1227–1239. - PubMed
-
- Wong TY, Cheung CM, Larsen M, Sharma S, Simo R.. Diabetic retinopathy. Nat Rev Dis Primers. 2016; 2: 16012. - PubMed
-
- Teo ZL, Tham Y-C, Yu M, et al. .. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021; 128: 1580–1591. - PubMed
-
- Vujosevic S, Aldington SJ, Silva P, et al. .. Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol. 2020; 8: 337–347. - PubMed
-
- Levine SR, Sapieha P, Dutta S, Sun JK, Gardner TW.. It is time for a moonshot to find “Cures” for diabetic retinal disease. Prog Retin Eye Res. 2022; 90: 101051. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

