New approach to busulfan dosing in infants and children based on a population pharmacokinetic analysis
- PMID: 39932505
- PMCID: PMC11813957
- DOI: 10.1007/s00280-025-04757-w
New approach to busulfan dosing in infants and children based on a population pharmacokinetic analysis
Abstract
Purpose: Apply population pharmacokinetic modeling to a single institution busulfan therapeutic drug monitoring (TDM) data set from infants and children to refine dosing methods.
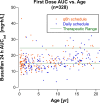
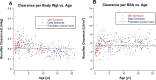
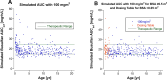
Methods: One-compartment pharmacokinetic model was fit to busulfan TDM data from 328 infants and children with malignant and non-malignant diseases treated with busulfan-containing transplant conditioning regimens. Age-dependence of busulfan clearance scaled to body weight and body surface area (BSA) was compared, and busulfan AUC was simulated for a BSA-scaled dose of 100 mg/m2 combined with a BSA-banded dosing table for infants and children with a BSA < 0.5 m2.
Results: Busulfan clearance scaled to body weight is age-dependent. Clearance in children ≤ 3 years (0.234 L/[h•kg]) is higher than the typical value for the population, (0.205 L/[h•kg]), and 48% of children < 5 years have subtherapeutic busulfan AUCs after the first dose. Busulfan clearance scaled to BSA (typical value, 5.47 L/[h•m2]) is more uniform across the pediatric age span, except for infants (≤ 1 year, 4.27 L/[h•m2]). Simulated busulfan AUCs with a dose of 100 mg/m2 for patients with a BSA ≥ 0.5 m2 combined with a BSA-banded dosing table for patients with a BSA < 0.5 m2 achieved a therapeutic AUC after the first dose in 49% more patients than body weight scaled doses.
Conclusion: Our model predicts a greater proportion of children would achieve a therapeutic busulfan AUC after the first dose with a dose of 100 mg/m2/d combined with the infant dosing table for patients with a BSA < 0.5 m2 compared to body weight-scaled dosing.
Keywords: Body surface area; Body weight; Busulfan; Dosing table; Stem cell transplantation; Therapeutic drug monitoring.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
References
-
- Bartelink IH, Lalmohamed A, van Reij EM, Dvorak CC, Savic RM, Zwaveling J, Bredius RG, Egberts AC, Bierings M, Kletzel M, Shaw PJ, Nath CE, Hempel G, Ansari M, Krajinovic M, Theoret Y, Duval M, Keizer RJ, Bittencourt H, Hassan M, Gungor T, Wynn RF, Veys P, Cuvelier GD, Marktel S, Chiesa R, Cowan MJ, Slatter MA, Stricherz MK, Jennissen C, Long-Boyle JR, Boelens JJ (2016) Association of Busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol 3(11):e526–e536. 10.1016/S2352-3026(16)30114-4 - PMC - PubMed
-
- Takahashi T, Jaber MM, Brown SJ, Al-Kofahi M (2023) Population Pharmacokinetic Model of Intravenous Busulfan in hematopoietic cell transplantation: systematic review and comparative simulations. Clin Pharmacokinet 62(7):955–968. 10.1007/s40262-023-01275-x - PubMed
-
- Pinkel D (1958) The use of body surface area as a criterion of drug dosage in cancer chemotherapy. Cancer Res 18(7):853–856 - PubMed
-
- Balis FM, Womer RB, Berg S, Winick N, Adamson PC, Fox E, Children’s Oncology Group Chemotherapy Standardization Task F (2017) Dosing anticancer drugs in infants: Current approach and recommendations from the Children’s Oncology Group’s Chemotherapy Standardization Task Force. Pediatr Blood Cancer 64(11). 10.1002/pbc.26636 - PubMed
-
- Morgan E, Baum E, Breslow N, Takashima J, D’Angio G (1988) Chemotherapy-related toxicity in infants treated according to the Second National Wilms’ Tumor Study. J Clin Oncol 6(1):51–55. 10.1200/JCO.1988.6.1.51 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

