Genetic insights into non-obstructive azoospermia: Implications for diagnosis and TESE outcomes
- PMID: 39932629
- PMCID: PMC12055743
- DOI: 10.1007/s10815-025-03409-5
Genetic insights into non-obstructive azoospermia: Implications for diagnosis and TESE outcomes
Abstract
Background: Non-obstructive azoospermia (NOA) is considered one of the most severe forms of male infertility. Despite the limited range of testicular phenotypes, NOA exhibits considerable genetic heterogeneity. The aim of this study was to uncover the etiopathogenesis of NOA and provide insights into the outcomes of testicular sperm extraction (TESE).
Material method: To elucidate the potential causes of testicular pathogenesis, a cohort of 61 patients was analyzed. The genetic etiology was assessed using our developed gene panel, based on genes with prior functional studies conducted specifically in the context of testicular characterization.
Results: Our analytical approach, built upon these findings, enabled us to explore the potential genetic causes of NOA and assess their relevance to TESE outcomes. A potential causal defect was identified in 14 genes across a total of 26 individuals (42%). Of these, three genes-MEIOB, TERB1, and USP26-had been previously described in men, while eight genes-SPO11, RBBP7, STS, RBMXL3, ZCCHC13, HUWE1, ESR1, and ABCD1-had been reported in prior studies. Additionally, three genes-CEP85, NAP1L3, and CENPI-had been previously described only in knockout (KO) phenotype studies, and this study represents the first identification of these genes in men.
Conclusion: Interestingly, the histological findings of meiotic arrest were strongly linked to genes involved in meiosis, reinforcing the clinical diagnosis of patients in this cohort. Additionally, our study underscores the importance of refining diagnostic strategies that focus on genes associated with testicular phenotypes, which could enhance the accuracy of TESE success predictions.
Keywords: Genetic heterogeneity; Meiotic arrest; Monogenic disorders; Non-obstructive azoospermia; TESE outcomes.
© 2025. The Author(s).
Conflict of interest statement
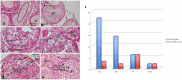
Declarations. Ethics approval: This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University (24/08/2022/ 1173610). Consent for publication: Informed consent was obtained from all the individual participants included in the study. The authors affirm that human research participants provide informed consent for publication of the images in Fig. 2a, b, c, d, and e. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

