Repeated Switching Between CT-P17 and EU Reference Adalimumab in Patients with Moderate-to-Severe Chronic Plaque Psoriasis: A Randomized, Double-Blind, Active-Controlled, Phase 3, Interchangeability Study
- PMID: 39932677
- PMCID: PMC11868361
- DOI: 10.1007/s12325-024-03100-8
Repeated Switching Between CT-P17 and EU Reference Adalimumab in Patients with Moderate-to-Severe Chronic Plaque Psoriasis: A Randomized, Double-Blind, Active-Controlled, Phase 3, Interchangeability Study
Erratum in
-
Correction to: Repeated Switching Between CT-P17 and EU Reference Adalimumab in Patients with Moderate-to-Severe Chronic Plaque Psoriasis: A Randomized, Double-Blind, Active-Controlled, Phase 3, Interchangeability Study.Adv Ther. 2025 Sep;42(9):4720-4723. doi: 10.1007/s12325-025-03235-2. Adv Ther. 2025. PMID: 40650708 Free PMC article. No abstract available.
Abstract
Introduction: This study aimed to demonstrate the interchangeability of biosimilar CT-P17 and European Union reference adalimumab (EU-adalimumab) in a repeated-switch scenario.
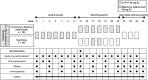
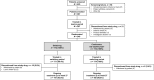
Methods: In this ongoing, randomized, double-blind, active-controlled, phase 3 study, adults with moderate-to-severe plaque psoriasis received 80 mg EU-adalimumab on day 1, then 40 mg 1 week later and every other week until week 11. At week 13, patients were randomized (1:1, via an interactive web response system) to continue EU-adalimumab ("continuous" group) or undergo repeated switches between CT-P17 and EU-adalimumab ("switching" group). Dosing was via subcutaneous administration. The primary endpoints were area under the concentration-time curve and maximum serum concentration between weeks 25 and 27 (AUCtau,W25-27 and Cmax,W25-27, respectively). Secondary endpoints comprised additional pharmacokinetic (PK) parameters, efficacy, safety, and immunogenicity. Week 27 findings are presented.
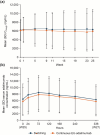
Results: The first patient provided signed informed consent on November 7, 2022. Week 27 visits were completed by August 14, 2023. Of 367 patients enrolled, 346 were randomized (switching group, n = 172; continuous group, n = 174). The ratios of least squares means between groups and associated 90% confidence intervals (CIs) for AUCtau,W25-27 and Cmax,W25-27 were 99.45% (94.11-105.08%) and 100.45% (95.03-106.17%), respectively. For both endpoints, 90% CIs fell within the predefined equivalence margin of 80-125% and criteria were greater than calculated t values, satisfying bioequivalence. Additional PK endpoints and efficacy, safety, and immunogenicity findings were similar between groups. Safety profiles were in line with those previously reported.
Conclusions: Week 27 primary PK results demonstrated bioequivalence, and the overall study results supported the interchangeability of CT-P17 and EU-adalimumab.
Trial registration: ClinicalTrials.gov, NCT05495568.
Keywords: Adalimumab; Biosimilar; CT-P17; Efficacy; Interchangeability; Pharmacokinetics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Mark G. Lebwohl is an employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara Therapeutics, Dermavant Sciences, Eli Lilly, Incyte, Inozyme, Janssen Research & Development, LLC, Ortho Dermatologics, Pfizer, Sanofi-Regeneron, and UCB; and is a consultant for Almirall, AltruBio Inc., AnaptysBio, Apogee, Arcutis Inc., AstraZeneca, Atomwise, Avotres Therapeutics, Brickell Biotech, Boehringer Ingelheim, Bristol Myers Squibb, Castle Biosciences, Celltrion, Corevitas, Dermavant Sciences, Dermsquared, EPI, Evommune Inc., Facilitation of International Dermatology Education, Forte Biosciences, Galderma, Genentech, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Sanofi-Regeneron, Seanergy, Strata, Takeda, Trevi, and Verrica. John Y. Koo is a consultant for Arcutis and Castle Pharmaceutical Corporation and is a speaker for AbbVie, Amgen, Bristol Myers Squibb, Dermavant, Eli Lilly, Janssen, Leo, Ortho-Dermatologic, Pfizer, Sanofi-Regeneron, and UCB. Paweł Brzewski has received consulting fees from AbbVie, Aristo, Eli Lilly, and Leo; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, and/or educational events from Eli Lilly, Leo, and Pfizer; payment for expert testimony from Avene, Cutis Lab, and La Roche Posay; support for attending meetings and/or travel from AbbVie, Leo, and UCB; has patents planned, issued, or pending from Leo; and is a member of EADV and a secretary for PDV Krakow. Marek Krogulec has received support for attending meetings and/or travel from Accord, Adamed, Belin-Chemie, Egis, Medac, and Sandoz. SungHyun Kim is an employee of Celltrion. YunJu Bae, DaBee Jeon, EunJin Choi, JungBin Cha, HyunJin Lee, and SuJin Choi are employees and stockholders of Celltrion, Inc. David M. Pariser has received consulting fees from Celltrion and has participated on a Data Safety Monitoring Board and/or Advisory Board for Celltrion. Janusz Jaworski, Jakub Trefler, Stefan Daniluk, Anna Dudek, Wojciech Baran, Witold Owczarek, Joanna Kolinek, and Mariusz Sikora have nothing to declare. Ethical Approval: All patients provided written, informed consent. The study was conducted in line with the principles of the Declaration of Helsinki 1964 (and its later amendments) and of Good Clinical Practice. Study protocols and documentation were approved by local independent ethics committees or institutional review boards at each study center (Table S1).
Figures
References
-
- AbbVie Inc. Adalimumab injection, for subcutaneous use US prescribing information. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/125057s423lbl.pdf. Accessed Oct 16, 2024.
-
- AbbVie Biotechnology GmbH. Humira summary of product characteristics. 2022. https://www.ema.europa.eu/en/documents/product-information/humira-epar-p.... Accessed Oct 16, 2024.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

