Loss of long-chain acyl-CoA dehydrogenase protects against acute kidney injury
- PMID: 39932791
- PMCID: PMC11949023
- DOI: 10.1172/jci.insight.186073
Loss of long-chain acyl-CoA dehydrogenase protects against acute kidney injury
Abstract
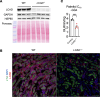
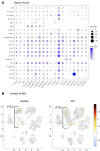
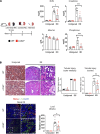
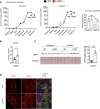
The renal tubular epithelial cells (RTECs) are particularly vulnerable to acute kidney injury (AKI). While fatty acids are the preferred energy source for RTECs via fatty acid oxidation (FAO), FAO-mediated H2O2 production in mitochondria has been shown to be a major source of oxidative stress. We have previously shown that a mitochondrial flavoprotein, long-chain acyl-CoA dehydrogenase (LCAD), which catalyzes a key step in mitochondrial FAO, directly produces H2O2 in vitro. Furthermore, we showed that renal LCAD becomes hyposuccinylated during AKI. Here, we demonstrated that succinylation of recombinant LCAD protein suppresses the production of H2O2. Following 2 distinct models of AKI, cisplatin treatment or renal ischemia/reperfusion injury (IRI), LCAD-/- mice demonstrated renoprotection. Specifically, LCAD-/- kidneys displayed mitigated renal tubular injury, decreased oxidative stress, preserved mitochondrial function, enhanced peroxisomal FAO, and decreased ferroptotic cell death. LCAD deficiency confers protection against 2 distinct models of AKI. This suggests a therapeutically attractive mechanism whereby preserved mitochondrial respiration as well as enhanced peroxisomal FAO by loss of LCAD mediates renoprotection against AKI.
Keywords: Cell stress; Fatty acid oxidation; Metabolism; Nephrology.
Figures




Update of
-
Loss of long-chain acyl-CoA dehydrogenase protects against acute kidney injury.bioRxiv [Preprint]. 2024 Oct 25:2024.10.22.619640. doi: 10.1101/2024.10.22.619640. bioRxiv. 2024. Update in: JCI Insight. 2025 Feb 11;10(6):e186073. doi: 10.1172/jci.insight.186073. PMID: 39484612 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

