Azithromycin as Host-Directed Therapy for Pulmonary Tuberculosis: A Randomized Pilot Trial
- PMID: 39932906
- PMCID: PMC12128081
- DOI: 10.1093/infdis/jiaf069
Azithromycin as Host-Directed Therapy for Pulmonary Tuberculosis: A Randomized Pilot Trial
Abstract
Background: Adjunctive host-directed therapies that modulate host immune responses to reduce excessive inflammation and prevent tissue damage in tuberculosis are being investigated. Macrolides, including azithromycin, were shown to possess anti-inflammatory and immune-modulatory effects in addition to their antibacterial effects. In the current trial, we investigated whether azithromycin enhances resolution of systemic and pulmonary inflammation and decreases extracellular matrix-related tissue turnover in tuberculosis patients.
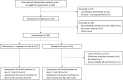
Methods: An open-label, randomized, controlled trial was performed. Adult patients with drug-susceptible, pulmonary tuberculosis aged above 18 years were randomly assigned to receive standard antituberculosis care or azithromycin 250 mg orally once daily in addition to standard care (SOC) for 28 days.
Results: Twenty-eight patients were included within 4 weeks after initiating antituberculosis treatment. Twelve patients in both arms completed the trial. Participants were mostly young, male, had a history of smoking, and had no comorbidities. No differences in baseline characteristics were observed between the study arms. In blood, azithromycin treatment significantly enhanced the reduction of the tuberculosis marker interferon-γ-induced protein-10 (SOC plus azithromycin, -38% vs SOC alone, -24% vs SOC, P < .05) and the collagen type IV degradation product C4M (-26% vs -11%, P < .05). In sputum, treatment with azithromycin significantly reduced neutrophils (-24% vs 0%, P < .001), neutrophil elastase (-88% vs 75%, P < .01), and transforming growth factor-β (-86% vs -68%, P < .05). No significant effects were observed on other parameters. Treatment with azithromycin appeared to be safe.
Conclusions: The addition of azithromycin to standard antituberculosis treatment appears to diminish excess neutrophilic inflammation in patients with pulmonary tuberculosis. Clinical Trials Registration. NCT03160638.
Keywords: azithromycin; extracellular matrix; host-directed therapy; inflammation; tuberculosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflict of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- World Health Organization . Global tuberculosis report. Geneva: World Health Organization, 2024.
-
- Allwood BW, Byrne A, Meghji J, Rachow A, Van Der Zalm MM, Schoch OD. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration 2021; 100:751–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

